Enology Notes #144
Enology Notes #144, October 2nd, 2008 - Link to PDF
To: Winemakers and Perspective Winemakers
From: Bruce Zoecklein, Professor and Head, Enology-Grape Chemistry Group, Virginia Tech
Subjects Discussed in Enology Notes #144:
- Wine Longevity and Aroma/Flavor
- Glutathione and Wine Longevity
- Timing of MLF
- New Virginia Tech Enology Service Lab Offering: Sanitation Monitoring
1. Wine Longevity and Aroma/Flavor.Wine longevity can be defined as the time period that the product conforms to stylistic goals. Crafting fine wine requires a holistic understanding of winemaking. Winemaking goals usually include the following (adapted from Delteil, 2008):
- No excess of SLO (sulfur-like off odors) impacting aromas and mouthfeel
- A stable and concentrated colloidal matrix
- No excess of volatiles contributing to “chemical” and “mineral” aromas
- No or limited herbaceous aromas
- No excess of harsh or “green” tannins
- Management of desirable aroma/flavors
a. Wine oxidation.Management of desirable aroma/flavor is a universal winemaking goal. Wine oxidation is characterized by the transformation of aroma/flavor compounds, leading to qualitative and quantitative changes resulting in a loss of wine-like fruity and estery aromas. On occasion, faulty aromas can develop, reminiscent of wax and naphthalene, as a result of ATA (atypical aging).
Table 1 (adapted from Nomacorc, 2008) illustrates a theoretical sensitivity of aroma compound descriptors to oxygen exposure.
Passion fruit |
Citrus |
Candy |
Sherry |
Oxygen Exposure
No Oxygen<------------------------------------------------------------------>More Oxygen
While descriptors such as bell pepper, grassy, chocolate, potato, earthy, flinty, and mushroom generally are not impacted by oxygen exposure (Nomacorc 2008), the mechanisms of oxidation are important to understand.
As illustrated in Figure 1, wine oxidation can involve the oxidation of a phenol to produce a quinone (oxidation product) and hydrogen peroxide (H2O2). In the example below, the hydrogen peroxide, a very strong oxidizing agent, oxidizes ethanol to acetaldehyde. This two-step reaction is referred to as coupled oxidation. Acetaldehyde can contribute to a sherry-like character.
Figure 1.
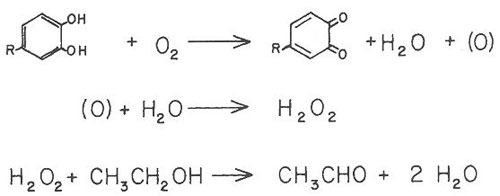
It is important to note that sulfur dioxide additions do not bind oxygen and, therefore, do not prevent the first step in this coupled oxidation.
b. Ascorbic acid. Some winemakers use ascorbic acid, or vitamin C, as an antioxidant added to juice and wine. Ascorbic acid sometimes protects the fruit characters and acts as an antioxidant while, at other times, it can act as a proto-oxidant, or oxidative promoter.
The two roles of ascorbic acid are mainly the result of concentration and the presence of adequate sulfur dioxide. As illustrated in Figure 2, when ascorbic acid is added to wine, it binds oxygen rapidly to form two reaction products, dehydroascorbate and hydrogen peroxide.
Figure 2.
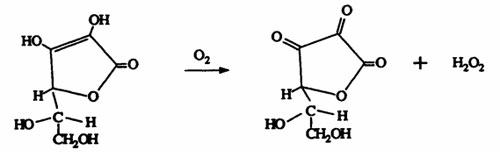
Ascorbic acid -------------------------> Dehydroascorbate + Hydrogen peroxide
If there is not enough ascorbic acid maintained to react with the oxygen, oxidation, including coupled oxidation (as illustrated in Figure 1) can occur. Additionally, if there is inadequate sulfur dioxide maintained to bind with the hydrogen peroxide formed by the ascorbic acid, wine oxidation can occur. The keys to optimizing the performance of ascorbic acid as an antioxidant are to maintain a concentration of about 50 mg/L, and to have adequate sulfur dioxide. Therefore, the use of ascorbic acid involves the following considerations:
- Reaction between ascorbic acid and oxygen is much more rapid than reactions involving (or with) SO2
- SO2 does not directly react with oxygen, but mainly with reaction products, such as H2O2
- Optimum levels of ascorbic acid (50 mg/L or more) and even more SO2 can prolong the antioxidant phase of ascorbic acid
- For example: If 100 mg/L ascorbic acid in wine reacts completely with oxygen, 62 mg/L SO2 is required to react with the ascorbic acid oxidation product
c. Metals and wine oxidation. Metals, such as iron or copper, have the disadvantage of being strong oxidizers, possibly impacting wine longevity. The potential oxidizing effect is illustrated by the Fenton-type reaction:
H2O2 + Cu+2 → Cu+3 + OH- + OH*
The OH*, or hydroxyl radical, is the most oxidative species. This is a potentially large problem, notably in white wines with relatively low concentrations of oxidative buffers such as phenols.
2. Glutathione and Wine Longevity. Glutathione (GSH) is a naturally occurring tripeptide and a strong antioxidant, as outlined in previous editions of Enology Notes (#98, 101, 102, 112, 127, 129, 134). The management of glutathione may be highly important for white wine longevity by helping to preserve aroma/flavor.
The enzymatic oxidation of a simple, but important juice phenol (caftaric acid) forms an oxidation product that can react with glutathione. This binding forms what is termed a grape reaction product (GRP). The grape reaction product terminates the oxidation process and subsequently limits oxidation (Cheynier, 2008).
The effect of this grape reaction product can be dramatic. For example, one molecule of a simple phenol can consume 3.4 atoms of oxygen. When this phenol is combined with glutathione to form the grape reaction product, that increases to 8.5 molecules (du Toit et al., 2007).
The following are some generalizations about glutathione (GSH) in white winemaking outlined, in part, by Laffort Inc. (2008).
- GSH increases with fruit maturation (Adam and Liyanage, 1993).
- The concentration is positively correlated to fruit YAN (yeast assimilable nitrogen) concentration (Dubourdieu and Lavigne-Cruege, 2004), another good reason for the analysis of YAN.
- Grape GSH concentration is easily lowered during processing. Attempts to limit this oxidation spurred the practice referred to as hyper-reduction, or anaerobic grape processing.
- GSH is assimilated by yeast during the beginning of fermentation and released at the end of alcoholic fermentation.
- Maximum release of GSH by yeast occurs only if yeasts have sufficient YAN (Laffort, 2008).
- 30 days-post fermentation, GSH levels can be as high or higher than in the initial juice (Dubourdieu and Lavigne-Cruege 2004).
- GSH can slow the decrease of some wine volatiles during aging (Roussis et al., 2007).
- The impact of GSH on limiting the decrease of volatile esters and terpenes during aging is concentration dependent (Papadopoulou and Roussis, 2008).
- It is estimated that 20 mg/L of GSH at the end of the aging period is optimum for aroma protection (Laffort, 2008).
- There is an inverse relationship between GSH and copper concentration in musts and wines (Dubourdieu, 2006).
- When lees are eliminated, the GSH concentration diminishes rapidly. In new barrels, this reduction is even greater due to the oxidation effect of new wood.
- Minimizing the loss of GSH is a key to optimizing white wine longevity.
- Some fermentation adjuncts contain glutathione.
a. Fermentation adjuncts and gluthatione. GSH is positively correlated to YAN, an additional justification for the analysis of YAN (previous editions of Enology Notes). Fermentation complements/addition products contain some or all the following:
- inorganic nitrogen (DAP)
- organic nitrogen (alpha amino acids)
- unsaturated fatty acids
- sterols, thiamine, folic acid, niacin, biotin and calcium pantothenate
- magnesium sulfate
- inactive yeast cell walls
- peptides
- micro-crystalline cellulose
- other yeast autolysis products, including GSH
One of the possible benefits of some fermentation complements/addition products is that they include GSH. Their use may help resist oxidation. Additionally, optimum oxygen, sulfur dioxide, pH, and lees management can increase the protection of white wine aromatic quality (see previous editions of Enology Notes at www.vtwines.info).
3. Timing of MLF. Currently, a large number of winemakers use selected cultures of lactic acid bacteria for the induction of MLF in wines. Most of these strains are species Oenococcus oeni.
The timing of MLF and the speed of the MLF completion have gained increased attention. In general, the earlier the MLF, the less evident are lactic aromas with more pronounced varietal aromas. Completion allows for the addition of sulfur dioxide to help limit oxidative breakdown of aroma compounds. This allows for a shorter time to have wines with limited sulfur dioxide, also possibly helping to control Brett growth.
4. Virginia Tech Enology Service Lab Offering: Sanitation Monitoring. The Virginia Tech Enology Service Laboratory has added sanitation monitoring to the list of available analyses. The purpose of this service is to allow winemakers to monitor the effectiveness of their sanitation procedures on tanks, barrels, winery equipment surfaces, bottling lines, etc. Such monitoring should be part of the winery HACCP procedures (see previous editions of Enology Notes). The Enology Service Laboratory will provide the necessary materials, which include environmental samplers and the directions for their use. Request forms and monitoring details are available at www.vtwines.info.
Table 2 below (adapted from Tracy and Skaalen, 2008) lists the numerous microorganisms that can impact wine, along with some of their sensory features. It should be understood that without proper monitoring, the effectiveness of a sanitation program is speculative.
Types of spoilage |
Wine microorganisms |
Excessive volatile acidity, primarily as acetic acid |
Brettanomyces, Candida, Kloeckera, Pichia, Zygosaccharomyces, Acetobacter, Lactobacillus, and Oenococcus spp. |
Ethyl acetate |
Candida, Kloeckera, Metschnikowia, and Pichia spp. |
Mousy taint |
Brettanomyces and Lactobacillus spp. |
Secondary fermentation in bottle |
Brettanomyces, Saccharomyces, Schizosaccharomyces, Zygosaccharomyces, Lactobacillus, Oenococcus, and Pediococcus spp. |
Yeast film on wine surface |
Candida and Pichia spp. |
Acetaldehyde formation |
Candida, Metschnikowia, Pichia, and Saccharomycoides spp. |
Diacetyl formation |
Lactobacillus and Pediococcus spp. |
Increased viscosity |
Pediococcus spp. |
Formation of ethyl carbamate precursors |
Saccharomyces, Lactobacillus, and Oenococcus spp. |
Formation of biogenic amines |
Brettanomyces, Candida, Kloeckera, Metschnikowia, Saccharomyces, Lactobacillus, Oenococcus, and Pediococcus spp. |
Formation of TCA (2,4,6-trichloroanisole) and TBA (2,4,6-tribromoanisole) |
Caused by various molds including Acremonium, Chrysonilia, Cladosporium, Fusarium, Penicillium, and Trichoderma spp. |
References
Adam, D. and C. Liyanage. 1993. Glutathione increases in grape berries at the onset of ripening. Am. J. Enol. Vitic. 44:333-338.
Cheynier, V. 2008. The discovery and characterization of grape reaction product and its role in must oxidative browning. Phenolic Substances in Grapes and Wines Symposium.
Delteil, D. 2008. Personnel communication.
du Toit, W. J., K. Lisjak, M. Stander and D. Prevoo. 2007. Using LC-MSMS to assess glutathione levels in South African white grape juice and wines made with different levels of oxygen. J. Agric. Food Chem. 55:2765-2769.
Dubourdieu, D. 2006. Personnel communication.
Dubourdieu, D. and V. Lavigne-Cruege. 2004. The role of glutathione on the aromatic evolution of dry white wine. Vinidea.net Wine Internet Technical Journal, 02. No 2.
Laffort Inc. 2008. Laffort. Bioarom. Natural aroma protection, natural reductive power.
Nomacorc Marketing Research. 2007. Research and post-bottling chemistry team, 2008. In: Does the choice of closure influence the sensory development of wines? Which closures are the most ‘green’? Nygarrd, M. 2008. Australian and New Zealand Grape Grower and Winemaker. August.
Papadopoulou D., and I.G. Roussis. 2008. Inhibition of the decline of linalool and alpha- terpineol in Muscat wines by glutathione and N-acetyl-cysteine. Int. J. Food Sci. 13:413-419.
Roussis, I., L. Lambropoulos and P. Tzimas. 2007. Protection of volatiles in wine with low sulfur dioxide by caffeic acid or glutathione. Am. J. Enol. Vitic. 58:274-278.
Tracy, R., and B. Skaalen. 2008. Wine microbiology. Practical Winery and Vineyard. Sept/Oct 83-86.
![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Enology-Grape Chemistry Group's web site at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes listserve send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce
Zoecklein
Professor and Enology Specialist Head Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Cell phone: 540-998-9025
Email:


