Enology Notes #134
Enology Notes #134, August 29, 2007
To: Regional Wine Producers
From: Bruce Zoecklein, Head, Enology-Grape Chemistry Group, Virginia Tech
Subjects Discussed in this Enology Notes:
3. Issues in Winery Layout and Design Workshop, March 7, 2008
4. France Trip, November 26-December 5, 2007
1. New Enology Notes Format. Due to formatting concerns, we have decided to use Adobe Acrobat for this and all future issues of Enology Notes. Our publication will arrive the same way in your email, but there will be an attached pdf file. The subjects included in the pdf file will be listed at the top of the page.
Please check to make sure that your email program does not automatically filter out messages with attachments.
Note – we will continue to post all Enology Notes on-line on our website for viewing, using your web browser, at www.vtwines.info.
2. Factors Impacting Sulfur-Like Odors in Wine, and Winery Options, Part 2. The following is adapted from a presentation I gave at the 8th Annual Enology and Viticulture British Columbia Wine Grape Council Conference, July 23-24, 2007 in Penticton, British Columbia, Canada. This is the conclusion. Part 1 appeared in Enology Notes #133.
Understand Post-Fermentation SLO Management Options. The following is a list and discussion of post-fermentation winery processing options for SLO management:
- Understand oxidation-reduction potential
- Monitor SLO
- Oxygen management, aeration, microoxygenation
- Antioxidants (sulfur dioxide, ascorbic acid)
- Lees management/yeast fining
- Tannin/silica additions
- Copper additions, copper impregnated pads
- Wine closures
A. Understand Oxidation-Reduction Potential.
Oxidation-reduction (redox) reactions describe the general principles and behavior of most wine chemistry. An understanding of redox is key to understanding SLO and their management. Some generalizations regarding redox include the following:
- Oxidation-reduction (redox) reactions are a series of interlinked reactions involving the oxidation of one compound and the reduction of another.
- Oxidation and reduction are two different chemical processes that complement each other.
- As electrons are transferred, one compound is oxidized, while the other is reduced.
- Oxidation is the loss of electrons. Reduction is the gain of electrons.
- For every oxidation reaction, there is a reduction reaction.
- Electrons are rearranging themselves into a more favorable order.
- This order is determined by the redox potential of the compounds.
- Oxygen is not required, although it is frequently the species that starts the process.
- Redox potential can be measured in the same way that pH is measured.
- Redox potential is a measure of how oxidative or reductive a system is, measured in millivolts (mV).
- The higher the mV, the less reductive, the more oxidative.
- Aerated red wine: redox potential 400-450 mV.
- Non-aerated stored red wine: 200-250 mV.
- Redox potential changes much more easily in whites.
- Tank wines have a lower redox potential than barrel wines.
- Redox potential is lowest at the bottom of a tank, hence the significance of lees stirring.
- Post-fermentation, a wine could be in a reduced state, but this does not necessarily mean that the wine is displaying SLO.
For additional information on redox potential see Enology Notes at www.vtwines.info.
B. Monitoring SLO.
Wine will always contain sulfide precursors, because these are normal constituents of fermentation, with an almost endless array of SLO in various states of oxidation and reduction. As a function of redox potential, these may manifest themselves in various forms post-fermentation.
The nature of the SLO compound(s) must be understood before remedial steps are taken. A sensory aroma screen should be conducted on all wines prior to bottling. The specific nature of such a screen is discussed in Zoecklein et al. (1999). It allows for the sensory separation of three general, but important, groups of SLO: hydrogen sulfide, thiols, and disulfides.
It is essential that winemakers conduct an aroma screen on all wines prior to any remedial SLO adjustments.
Table 1. Outline of a sensory aroma screen for determining the general nature of some SLO in wine.
Control |
Copper Sulfate |
Cadmium Sulfate |
Interpretation |
Presence of offensive odor |
Odor is gone |
Odor is gone |
H2S present |
Odor is gone |
No change |
Mercaptans |
|
Odor is gone |
Odor is less, but not gone |
Both H2S and mercaptans |
|
No change |
No change |
Dimethyl disulfide or other |
Three glasses of the same wine are evaluated: a control, a glass containing copper sulfate, and a glass containing cadmium sulfate. The interpretation is given in Table 1. Note: This review is solely for odor evaluation: these wines should not be tasted.
C. Wine Aeration/Oxygenation.
One misconception is the belief that a wine with SLO can always be fixed by oxidation. This has arisen due to some sensory changes noted on some occasions. If H2S is present, the oxidation of H2S to elemental sulfur can occur as below:
2H2S + O2 <--> 2H2O +2 S
This leaves elemental sulfur present at the bottom of a tank, which must be removed. Otherwise, the reverse reaction would occur. While this reaction can occur, wines contain a host of antioxidants or reducing agents that will compete for any oxygen added.
There is some volatilization of low boilers like H2S that can occur. How much volatilization is possible depends upon the particular SLO compound(s). What does occur with oxygen exposure is that the form of the sulfide changes, in accordance with the shift in the redox potential.
Figure 1.
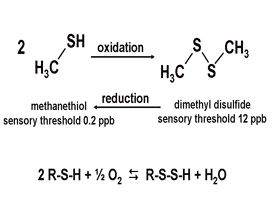
A good example of redox is the oxidation of methanethiol to dimethyl disulfide. Oxidation of one SLO compound to a slightly less stinky one is sometimes possible. The sensory thresholds for sulfides shift markedly with small changes in molecular structure, ranging from 2 ppb to 12 ppb. Note that no oxygen is involved. One compound, like methanethiol, can be oxidized to form dimethyl disulfide. This reaction is reversible.
Oxidation of methanethiol to disulfides can easily take place with wine aeration. Aeration may not remove sulfides, but simply change their form and, therefore, their sensory descriptor and thresholds. Oxidation causes a cascading set of reactions stabilizing the electron shifts. The redox potential would be readjusted to near, but not exactly the same, as the original potential. To help avoid unwanted oxidation, especially in white wines, H2S may be blown off with inert gases, such as nitrogen. However, this may take a significant quantity of gas and requires an understanding of the specific SLO in the wine, e.g. an aroma screen.
Microoxygenation. It has been known for some time that microoxygenation can lower the perception of veggie/herbal character in a wine. Originally, we presumed this effect was the result of changes in pyrazines. However, that was not confirmed by our analysis. The odor of thiols complements those of pyrazines and indeed some thiols contribute to “green”-type odors. Microoxygenation results in oxidation of some thiols, resulting in both a change in the perception of SLO and veggie character as noted below. This highlights the interrelationships of aroma compounds in wines.
Figure 2
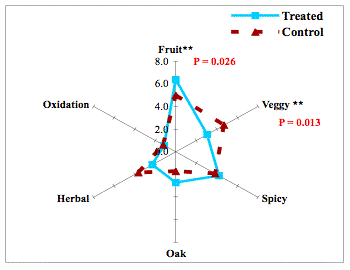
D. Copper Addition.
The use of copper poses an interesting dilemma to winemakers. It can be used to treat H2S and some thios but, at the same time, will reduce the concentration of desirable VSC compounds. It does not discriminate between SLO and VSCs contributing to varietal character. Some of the considerations regarding the use of copper include the following:
- Legality/perception
- Reactivity only with certain SLOs
- Protein haze
- Timing of addition: yeast stress, redox
- Sensory impact on varietal character and intensity
- Impact on longevity
Copper can react with some SLO, while not others:
- H2S and thiols react with Cu+2
- Disulfides and thioesters do not react with Cu+2
- Thioesters can degrade to thiols (and esters), which can react with Cu+2
Copper reacts with hydrogen sulfide according to the following reaction:
H2S + CuSO4 → CuS + H2SO4
Copper also reacts with some thiols, including methyl mercaptan. However, copper does not react with disulfides (thiol oxidation product).
2 CH3SH + ½ O2 → CH3SSCH3 + H2O
Methyl mercaptan dimethyl disulfide
SO2
Ascorbic Acid
←
Copper sulfide will not react with disulfides or heavy sulfur compounds. However, copper sulfide has been used with sulfur dioxide and ascorbic acid, whereby the SO2 cleaves the disulfide, resulting in two mercaptans which can then be bound with copper.
The ascorbic acid acts as an antioxidant to keep the mercaptan from oxidizing. One of several problems is that this reaction is very slow at wine pHs. The above reaction illustrates the importance of conducting aroma screens.
In addition to only reacting with certain SLO, copper also has the disadvantage of being a strong oxidizer, possibly impacting wine longevity. The potential oxidizing effect is illustrated by the Fenton-type reaction:
H2O2 + Cu+2 → Cu+3 + OH- + OH*
The OH*, or hydroxyl radical, is the most oxidative species. This is a potentially large problem, notably in white wines with relatively lower concentrations of oxidative buffers, such as phenols.
Addition of copper sulfate to the fermentor is a practice used by some in an attempt to limit SLO production. While the majority of the copper (about 60% or more) is bound to yeast and precipitates from solution, such additions are not benign. Copper addition, either during or post-fermentation, can have a large negative impact by lowering the varietal intensity of the aromas derived from VSC. As such, the varietal characters of Sauvignon blanc, Riesling, Gewürtztraminer, Petit Manseng, and Chenin blanc, are diminished due to copper’s ability to bind mercapto- compounds.
Copper and Glutathione. Copper also impacts wine longevity as a result of oxidation and removal of antioxidants. One of the most important antioxidants in white wines is glutathione. Glutathione is a sulfur-containing polypeptide both found in grapes and produced by yeasts. It is a strong antioxidant. As such, it helps protect labile aroma/flavor compounds from oxidative degradation. Copper additions, in the form of Bordeaux sprays and as a remedial winemaking activity, have the ability to bind and completely inactivate glutathione. Optimizing the production and management of glutathione may be the key to winemaking of low phenol whites. See Enology Notes Index for additional information on this subject at www.vtwines.info.
E. Ascorbic Acid and SLO Management.
Understanding the mechanisms of oxidation is important. As illustrated in Figure 3, wine oxidation can involve the oxidation of a phenol to produce a quinone (oxidation product) and hydrogen peroxide. In the example below, the hydrogen peroxide generated oxidizes ethanol to acetaldehyde (coupled oxidation).
Figure 3.
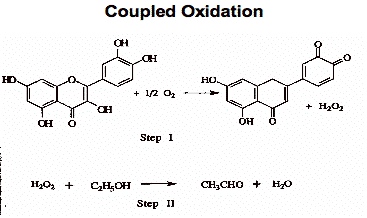
It is important to note that sulfur dioxide additions do not bind the oxygen and, therefore, do not prevent the first step in this coupled oxidation. Some winemakers use ascorbic acid, or vitamin C, as an antioxidant. Ascorbic acid sometimes protects the fruit and acts as an antioxidant, while at other times it can act as a protooxidant, or oxidative promoter.
The two roles of ascorbic acid are mainly the result of concentration and the presence of adequate sulfur dioxide. As illustrated below from Zoecklein et al, 1999, when ascorbic acid is added to wine, it binds oxygen rapidly to form two reaction products, dehydroascorbate and hydrogen peroxide. If there is not enough ascorbic acid maintained to react with the oxygen, oxidative degradation, including coupled oxidation, can occur. If there is not adequate sulfur dioxide maintained to bind with the hydrogen peroxide formed by the ascorbic acid, wine oxidation can occur.
Figure 4.
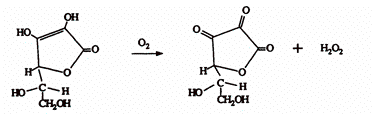
Therefore, the keys to optimizing the performance of ascorbic acid as an antioxidant are to maintain a concentration of about 50 mg/L and to have adequate sulfur dioxide. Therefore, the use of ascorbic acid involves the following considerations:
- Reaction between ascorbic acid and oxygen much more rapid than SO2
- SO2 does not directly react with oxygen, but mainly with reaction products, such as H2O2
- Optimum levels of ascorbic acid (50 mg/L or more), and more SO2 can prolong the antioxidant phase of ascorbic acid.
- For example: If 100 mg/L ascorbic acid in wine reacts completely with oxygen, 62 mg/L SO2 is required to react with the ascorbic acid oxidation product
F. Wine Closures and SLO.
Wine closures can impact post-bottling SLO. The following are important considerations:
- Low or no oxygen ingress screw cap-type closures/liners are more prone to cause accumulation of thiols post-bottling
- Low oxygen ingress results in a lowering of the redox potential
- Lack of oxygen to oxidize thiols to disulfides can impact SLO perception
- To deal with this potential problem, some are adding copper at bottling
- Cu+2 bottling can impact longevity, but can bind H2S and some thiols
- Copper addition at bottling has no impact on disulfides and thiolesters
3. Issues in Winery Layout and Design Workshop. Dr. Bruce Zoecklein, Head, Enology-Grape Chemistry Group, Virginia Tech, coordinator.
March 7, 2008, King of Prussia, PA. This program is associated with the 2008 Wineries Unlimited program. Registration information will be available shortly at www.vwm-online.com/wu.
This one-day program will cover practical topics of interest to those establishing a new winery, or expanding an existing facility. Industry members and winery architects will discuss and review the following:
Winery Design
- Winery Facility Design Concepts
- Construction Process Map
- Winery Design Considerations
- Winery Layout
- Winery Tasting Room Design Issues
Examples of Winery Designs
- Winery Architecture
- Winery Designs and Case Studies
Integration of Winery Process Equipment, Layout, and Design
- Stylistic Winemaking and Winery Design
- Equipment Considerations Overview
- Equipping Small vs. Large Wineries
- Fermentation and Storage Vessel Considerations
- Wine Caves
- Gravity Flow Winery Designs
Green Winery Design Considerations
- Winery Sustainability Options
- Saving Energy and Water
4. French Wine Study Tour. A wine study tour is planned for November 26 to December 5, 2007.
The group will experience the new terroirs in the hills of Languedoc (modern blended varieties), the wines of Listel, the success of Mourvedre and Viognier in the “Montagne Ste Victoire” around Aix en Provence, the new fashion for rosé wines in the rolling hills of Provence, the rare Tiburen grape variety, and finish the study tour in Nice. The tour will also include a day at SITEVI, the equipment show in Montpellier.
The itinerary is as follows.
- Leave the US on Monday, November 26, 2007 for Montpellier, France (we hope to have all participants depart together from Washington-Dulles).
- Arrive at Montpellier airport Tuesday morning – to Hotel.
- Tuesday afternoon, November 27 and Wednesday, November 28 – Western Languedoc: Focus on new styles of Languedoc wines (varieties, techniques) with stops at Skalli-Sète (Fortant de France), Institut Coopératif du Vin (experimental labs), Coop-Florensac (new blends) and Mas-de-Saporta (South of France Wine Alliance).
- Thursday, November 29 – SITEVI at Montpellier: Focus on new equipment for small wineries. In the afternoon, stop at Listel-Vin des Sables along the sea.
- Friday, November 30 - Eastern Languedoc: Focus on successful wines of the Southern East Bank of the river Rhône at Beaucaire and Tavel. Stop at Arles.
- Saturday, December 1 - The Left Bank: gate of Provence: Matching Mediterranean food and wines in the foothills of Alpilles and Luberon, from the antique city of Les Baux to Aix en Provence, along the olive trees. Stop at Château de Beaulieu, a key player in the new Provence styles.
- Sunday, December 2 – The Appellations of Provence 1: A diversity of "terroirs". Visit two wineries along the beaches at Bandol and Lalonde Les Maures producing whites (Rolle, Ugni blanc, Clairette, Grenache blanc, Sauvignon blanc, Semillon and Marsanne), rosés (Tiburen) and reds (Mourvedre, Grenache, Syrah, Cinsault, Carignan and Cabernet franc).
- Monday, December 3 – The Appellations of Provence 2: Focus on the rosé phenomenon. Day with the Association of Producers of Provence, visit and tasting at the new Experiment center and at Château St Martin. Last tasting late afternoon at St Tropez, the hotspot for the European jetset.
- Tuesday, December 4 – Back to the origin: At St Honorat, tour of the first vineyards settled by the Greeks twenty-five hundred years ago, nestled along a monastery since the Middle Ages. Relax or some shopping in the afternoon. Goodbye dinner at a winery (Folle Noire and Braquet) around Nice. End of the tour.
- Wednesday, December 5 – Depart from Nice airport (or additional tour on your own along the French Riviera).
General schedule
Dates:
In France from Tuesday morning, November 27th at Montpellier Airport to Wednesday morning, December 5th at Nice Airport.
Air Transportation (not included):
The most convenient is Air France, leaving Washington-Dulles Monday afternoon, November 26th, with a direct connection at Paris-Charles de Gaulle airport to Montpellier (direct reservation). Same direct connection on the way back from Nice, via Paris-Charles de Gaulle airport. We hope to travel as a group to France, but this is not required.
General conditions for the tour
Price includes
- all domestic transportation in France, from airport to airport, by Pullman bus
- three-star hotels
- beverages and meals, visits, tastings, translation, and documents
Not included:
- the round trip air ticket from the US
- telephone calls, extra beverages, and other personal expenses during the tour
For a single room: 2,300 Euros per person
For couples or sharing a room: 2,100 Euros per person
Registration. Registration is on a first-come first-served basis. Space is reserved only after the receipt of your non-refundable deposit check of $250 per person, payable to Bruce Zoecklein, and mailed to Bruce Zoecklein, Department of Food Science and Technology (0418), Virginia Tech, Blacksburg, Virginia 24061. Any questions can be directed to me at 540-231-5325 or email at .
You must provide the following with your registration payment:
- Name
- Complete address
- Home phone number
- Daytime phone number
- Email address
- Passport number
Registration must be received by October 31, 2007.
This study tour has as maximum limit of 20 people.
For added insight to the nature of these study tours, check On-Line Publications, France Study Tour 2002, or click Enology Notes Index, France, both at www.vtwines.info. To date, four trips to France have been organized.
![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Enology-Grape Chemistry Group's web site at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes listserve send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce
Zoecklein
Professor and Enology Specialist Head Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Cell phone: 540-998-9025
Email:


