Enology Notes
Enology Notes #133, August 14, 2007
To: Regional Wine Producers
From: Bruce Zoecklein, Head, Enology-Grape Chemistry Group, Virginia Tech
Subjects: Web Resources; Oxidation and Ascorbic Acid; Pre-Harvest and Harvest Fermentable Nitrogen Analysis; Factors Impacting Sulfur-Like Off Odors in Wine, and Winery Options, Part 1; France Trip; New Edition of Winery Planning and Design CD
1. Web Resources. A number of harvest and post-harvest resources are posted on the Enology-Grape Chemistry Group’s website at www.vtwines.info. Click Enology Notes and then Enology Notes Index or On-Line Publications.
2. Oxidation and Ascorbic Acid. Understanding the mechanisms of oxidation is important. As discussed by Dominique Delteil in his excellent presentation on wine longevity, how wines develop in the bottle should be carefully reviewed prior to the season to determine if production practices should be adjusted. As illustrated in Figure 1, wine oxidation can involve the oxidation of a phenol to produce a quinone (oxidation product) and hydrogen peroxide (H2O2). In the example below, the hydrogen peroxide generated oxidizes ethanol to acetaldehyde (coupled oxidation).
Figure 1.
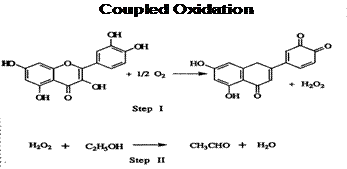
It is important to note that sulfur dioxide additions do not bind the oxygen and, therefore, do not prevent the first step in this coupled oxidation. Some winemakers use ascorbic acid, or vitamin C, as an antioxidant. Ascorbic acid sometimes protects the fruit and acts as an antioxidant, while at other times it can act as a proto-oxidant, or oxidative promoter.
The two roles of ascorbic acid are mainly the result of concentration and the presence of adequate sulfur dioxide. As illustrated below, when ascorbic acid is added to wine, it binds oxygen rapidly to form two reaction products, dehydroascorbate and hydrogen peroxide. If there is not enough ascorbic acid maintained to react with the oxygen, oxidative degradation, including coupled oxidation, can occur. If there is not adequate sulfur dioxide maintained to bind with the hydrogen peroxide formed by the ascorbic acid, wine oxidation can occur.
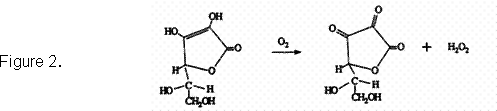
Therefore, the keys to optimizing the performance of ascorbic acid as an antioxidant are to maintain a concentration of about 50 mg/L, and to have adequate sulfur dioxide. The use of ascorbic acid involves the following considerations:
- Reaction between ascorbic acid and oxygen much more rapid than SO2
- SO2 does not directly react with oxygen, but mainly with reaction products, such as H2O2
- Optimum levels of ascorbic acid (50 mg/L or more) and more SO2 can prolong the antioxidant phase of ascorbic acid.
- For example: If 100 mg/L ascorbic acid in wine reacts completely with oxygen, 62 mg/L SO2 is required to react with the ascorbic acid oxidation product
The possible use of ascorbic acid should be determined based on the assessment of white wine longevity and oxidation potential. This addition compound may have a place in the production of some delicate, low phenol white wines.
3. Pre-Harvest and Harvest Fermentable Nitrogen Analysis.
- Email the Laboratory at to request processing bags and bottles
- Include name, company, mailing address, and the number of sampling kits required
- Collect juice samples at harvest, or grape berry samples from the vineyard pre-harvest*
- Fill sample bottles to the indicated level, mix thoroughly to dissolve preservative
- Ship samples overnight to the Laboratory; shipping delays impact analysis results
*Complete information regarding sampling and berry-bag processing can be found on the VT Enology-Grape Chemistry Group website (www.vtwines.info) under Online Publications > Maturity Evaluation for Growers.
The results are strongly dependent on adequate and representative sampling in the vineyard and proper sample processing.
Fermentable Nitrogen Analysis - $25
Ship samples to:
Wine/Enology-Grape Chemistry Group
Enology Service Lab
Attn: Ken Hurley
Rm. 113, FST Bldg.
Virginia Tech (0418)
Blacksburg, VA 24061
For more information on available analyses and analytical panels, please see the Enology-Grape Chemistry website at www.vtwines.info.
4. Factors Impacting Sulfur-Like Off Odors in Wine, and Winery Options, Part 1. The following is adapted from a presentation given at the 8th Annual Enology and Viticulture British Columbia Wine Grape Council Conference, July 23-24, 2007 in Penticton, British Columbia, Canada.
Crafting fine wine requires a holistic understanding of winemaking, and usually includes the following goals:
- Maintain a stable and concentrated colloidal matrix
- No excess of volatiles contributing to “chemical” and “mineral” aromas
- No or limited herbaceous aromas
- No excess harsh or “green” tannins
- Management of desirable varietals, including volatile sulfur compounds (VSC)
- No excess sulfur-like odors (SLO) impacting aromas and mouthfeel
Managing VSC represents a double-edged sword. On the one hand, certain sulfur-containing compounds, like H2S, can contribute to SLO and impart negative attributes; on the other hand, some sulfur compounds, like 3-mercaptohexanol and 3-mercaptohexylacetate (3-MHA), impart fruitiness and have a positive aroma impact. Furthermore, VSC compounds can become more or less desirable, depending upon their absolute concentration, their relative concentration, and the specific wine matrix. Therefore, the challenge for winemakers is to modulate the concentrations of VSC in accordance with consumer preferences and stylistic goals.
Understanding SLO requires an integration of academic findings, empirical knowledge and practical in-house experimentation. It is hoped that this review will aid in that integration.
Yeast Production of SLO. Yeasts can utilize elemental sulfur, sulfate, sulfide, sulfite, and organic sources of sulfur in grape juice to produce H2S:
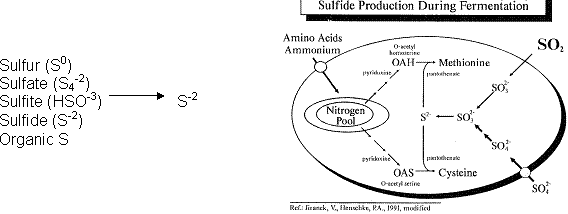
As a product of sulfate reduction, H2S is an intermediate in the biosynthesis of all sulfur-containing compounds required for cell growth and function.
SLO formation in wine is governed by the many factors that influence the yeast sulfide reduction system. As illustrated above, in a series of regulated steps, sulfate is brought into the cell and reduced to sulfide via two ATP-activation steps. At this point, sulfide is combined enzymatically with nitrogen-containing carbon precursors to ultimately form cysteine and methionine, two S-containing amino acids. This sulfate reduction sequence is activated to produce sulfide whenever there is a metabolic demand for cysteine and methionine. All organic sulfur compounds are formed via sulfur-containing amino acids. In the absence of intracellular nitrogen, this reduction sequence can continue, forming excess H2S which is not incorporated into amino acids, but is liberated into the wine. Therefore, a high rate of sustained H2S production can be observed in response to N deficiency.
Sulfite (sulfur dioxide added pre-fermentation) freely diffuses into the cell, as illustrated. As such, it essentially bypasses the regulatory mechanisms normally controlling sulfate reduction. This helps to explain why H2S production can be greatest when too much sulfite (more than 80 mg/L, depending upon the yeast strain) is present during fermentation.
SLO Compounds Commonly Found in Wines. There are nearly 100 VSCs reported in wine, some desirable, others contributing to SLO.
Table 1. Examples of Sulfur-like Off Odor Compounds in Wines
SLO |
Sensory Description |
Sensory Threshold (μg/L) |
Boiling Point (ºC) |
Hydrogen Sulfide |
Rotten egg |
0.5 |
-61 |
Carbonyl sulfide |
Ether |
3.0 |
-50 |
Methyl Mercaptan, Methanethiol |
Stagnant water |
1.5 |
6 |
Ethyl Mercaptan, |
Onion |
1.1 |
35 |
Dimethyl Sulfide |
Quince, truffle |
10.0 |
35 |
Methionol |
Cooked cabbage |
1200 |
90 |
Diethyl Sulfide |
Ether |
0.9 |
92 |
Dimethyl Disulfide |
Quince, asparagus |
15.0 |
109 |
Diethyl Disulfide |
Garlic, rubber |
4.3 |
151 |
Thioesters |
|||
Source: Swieger et al. (2006)
As pure compounds, the above have different sensory characteristics and, generally, low to very-low sensory thresholds. Beyond these differences, SLO can be divided into three categories: light or low-boiling-point compounds – those with a boiling point below 90°C; heavy or high-boiling-point sulfur compounds – those with a boiling point above 90°C; and thiol-acetic acid esters. This is not an academic distinction; the light compounds are produced during and post-fermentation, and have unpleasant odor descriptors. Because of their volatility, their concentration may be lowered by aeration or sparging. Some members of this group (H2S and mercaptans) react with copper.
The heavy, or high-boiling-point compounds are produced by yeast metabolism during fermentation, but not post-fermentation. These remain stable in the wine post-fermentation. High boilers cannot be removed by aeration due to their limited volatility, and they do not react with copper. As such, they represent a large winemaking problem.
The thioesters are odorless, but can undergo hydrolysis, or breakdown, to release thiols, thus contributing to disagreeable odors.
Sensory Features of SLO. The sensory attributes of SLO listed above change as a function of concentration (absolute and relative), and with the nature of the wine matrix. For example, a thiol that has an attribute of peas or vegetal at low concentrations, may be described as rotten cabbage, etc., at higher concentrations. Evaluations for SLO in wines should be conducted on all wines pre-bottling.
Most think of SLO in terms of olfactory sensations alone. However, SLO can have an impact on wine mouthfeel, imparting a mineral, bitter, hard, and/or astringent aspect. (For additional information on this subject, see www.vtwines.info. Under Industry Pubs, click On-line Publications, then Components of Red Wine Mouthfeel.)
It is the quantitative and qualitative nature of SLO, such as those listed in Table 1, that provides the sensory impression and dictates remedial winemaking actions.
Factors Impacting SLO Formation. The following is a partial list of the many factors that may contribute to the production of SLO by yeast:
- Elemental sulfur
- Presence of high levels of sulfur dioxide
- Degradation of sulfur-containing amino acids
- Release and/or metabolism of grape-derived sulfur-containing precursors
- Nutritional deficiency
- Nitrogen
- Oxygen
- Pantothenate
- High threonine, relative to other amino acids
- Relative methionine-to-ammonia concentrations
- Yeast stress
- Yeast genetics
Practical SLO Management. The following is a list and discussion of practical winemaking steps to consider in SLO management:
- Understand factors impacting yeast performance
- Measure yeast assimilable nitrogen
- Understand viticultural and environmental factors impacting fermentable N
- Fruit processing and assimilable N
- Control must turbidity
- Proper concentration and timing of nutrients
- Optimize oxygen management
- Avoid carbon dioxide toxicity
- Understand oxidation-reduction potential in regards to SLO
- Understand post-fermentation and SLO
- Have a HACCP-like plan
A. Understanding Yeast Performance.
Figure 3.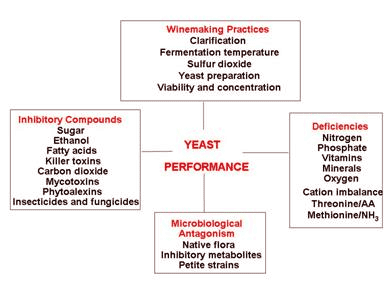
Gump, Zoecklein and Fugelsang (2001)
Figure 3 illustrates the vast array of factors that influence yeast performance. Many of these contribute to yeast stress, individually or collectively. Stress can change the biochemical machinery, possibly resulting in a reduction in fermentation capacity, and premature cell death. Premature cell death results in autolysis, with the release of sulfur-containing amino acids and peptides. Conditions that produce yeast stress and unhealthy cells are more likely to promote autolysis and increase the production of SLO.
B. Measure Fermentable Nitrogen.
Fermentable nitrogen has received considerable attention because it can be a contributor to SLO production, both too high and too low a concentration. Additionally, N is relatively easy to measure.
The nitrogen required by yeast to conduct a healthy fermentation includes two forms, ammonia N, and a group of amino acids referred to as alpha-amino acids, or free amino nitrogen (FAN). Together, both sources contribute the nitrogen utilized by yeast, referred to as yeast-assimilable (YAN), or fermentable nitrogen.
The minimum yeast assimilable nitrogen required is approximately 140 mg/L for a 21-degrees Brix juice, and perhaps 250 mg/L for a 23-degrees Brix juice. However, it should be noted that these concentrations are broad-based generalizations for several reasons:
- The nitrogen level requirement to optimize fermentation is highly yeast-strain specific, governed largely by the genetics of the yeast.
- It may be that the qualitative makeup of FAN amino acids, not simply the total yeast assimilable N, is the most important factor. The significance of the qualitative nature of YAN helps to explain so-called reductive grapes, varieties that have a greater tendency to produce SLO. It also helps to explain seasonal and block differences in SLO production.
- A low concentration of assimilable nitrogen is often coupled with deficiencies in important micronutrients.
Two common procedures for measurement of fermentable N are the Formol titration and NOPA test. We modified the Formol titration procedure and compared the results with the NOPA method (Am. J. Enol. Vitic. 53:325-329). Some features of these two procedures are summarized below:
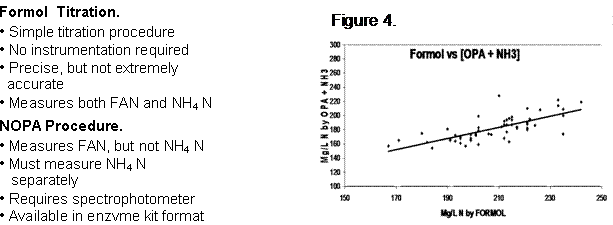
The Formol titration has the advantage of measuring both ammonia and FAN amino acids. However, the method over-titrates proline (which yeast cannot use) and under-titrates arginine, which the yeast do use. Our research suggested that this generally balances out. Arginine accumulation begins well before véraison and continues to maturity, then plateaus. Proline, on the other hand, increases late in the season (four weeks post-véraison). High proline is associated with increased maturity and with vine stress.
Figure 4 shows the relationship between the two tests. As can be seen, the methods are comparable, demonstrating reasonable linearity. We have developed a low-volume Formol procedure which is currently on my website (www.vtwines.info, under Industry Pubs, click On-line Publications, then Reduced Volume Formol Titration, adjacent to Fermentable Nitrogen).
C. Understand Viticultural and Environmental Factors Impacting Assimilable N.
A number of viticultural and environmental factors can impact yeast assimilable N, including the following:
- Cultivar
- Crop Load
- Rot Incidence
- Moisture Stress
- Block
- Maturity Level
(For a discussion of these subjects, see www.vtwines.info, under Industry Pubs, click Enology Notes, then Subject Index to Enology Notes.)
From véraison onward, the following changes occur in the fruit:
- NH3 increases, then declines
- FAN amino acids increase, then decline, with the rate of decline different among FAN components
- With extended maturity, YAN declines
There appear to be some correlations between the quantitative changes in YAN and SLO formation, including high threonine, relative to other amino acids, and the relative methionine-to-ammonia concentration. Both situations are reported to contribute to SLO formation. There also appears to be a correlation between ATA (atypical aging) and SLO formation (See Enology Notes #110).
Some generalizations regarding cultivars and YAN are listed below. It should be noted, however, that site, season and vineyard management practices can have a very large influence.
- Merlot: usually low in YAN
- Syrah: usually somewhat low in YAN; this is coupled with high potential alcohol
- Pinot Noir: often sufficient in YAN
- Sauvignon Blanc: often sufficient in YAN
D. Understand Grape Processing and YAN.
There is a relationship between juice extraction methods and fermentable nitrogen. This relationship stems from the fact that arginine, the FAN amino acid in the greatest concentration, is located mainly in the skins. Therefore, winemaking protocols, such as the following contrasts, result in different YAN concentrations:
- Whole cluster pressing vs. crush and drain of whites
- Bleeding vs. non-dejuiced reds
- Short vs. long-vatted reds
For those evaluating the N status of vineyard samples just prior to harvest, which I recommend, the relationship between sample processing methodology and cellar processing must be noted.
E. Control the Non-Soluble Solids (NSS) Level.
Turbidity of white juice should be adjusted with some precision, to attain stylistic goals and the aromatic finesse of the wine. Juice clarity can be measured in nephol units (NTU). The desirable NTU range is between 100 and 250. Low non-soluble solids concentration going into the fermentor can result in a low concentration of YAN and other nutrients, and can increase the likelihood of SLO. High NSS concentration increases the risk of SLO production, including high boiling compounds.
F. Use Optimum Oxygen Management.
Yeast produce membrane lipids only when grown aerobically. In the initial growth phase, proper oxygen management leads to proper production and storage of sterols in the yeast cell, which can be shared with subsequent daughter cells. It is possible to increase yeast ethanol tolerance by promoting synthesis of sterols, by adding oxygen (air) in the starter and during fermentation. Yeast lees deplete the oxygen content and can impact the redox potential and formation of SLO.
Additionally, some yeast-derived commercial products aid in sterol synthesis. Oxygen management involves an understanding of the following:
- Optimum 8-10 mg/L oxygen during the initial growth phase
- Oxidative stress may be a primary cause of early yeast mortality
- Lees are potent oxygen consumers, even after yeast cell death
- Lack of oxygen can contribute to SLO
- Oxygen additions may allow yeast to produce more glutathione, an important white wine antioxidant
G. Proper Yeast Selection.
Wine yeast play a central role in the production of volatile sulfur compounds, both the good and the bad. Yeast are responsible for the transformation of non-volatile grape-derived precursors to odor-active volatiles, which can positively contribute to thiol-based varietal character of a number of cultivars, including Sauvignon blanc, Chenin blanc, Riesling, and Petite Manseng. Wine yeasts vary tremendously with regard to this conversion.
SLO production is controlled more by yeast genetics than winemaking, however fermentation environment does play a role. For example, the level of H2S can vary by as much as 2000-fold for a given strain, simply by changing the environment.
Some strains are less efficient users of nitrogen and have higher nitrogen requirements. Commercial strains can vary by more that 50% with regard to their N requirement. Additionally, uninoculated, feral fermentations, with a large population of non-Saccharomyces cells (therefore not alcohol tolerant) can cause problems. Non-Saccharomyces become inhibited by the increasing alcohol concentration, lose viability and undergo autolysis during the early- to mid-stages of the alcoholic fermentation.
H. Proper Concentration and Timing of Supplements.
Improper concentration and timing of N supplements can result in the following:
- Increased production of SLO
- Increased unwanted flora (if added too early or too late)
- Rapid fermentation
- Loss of volatiles, particularly if the source is DAP
- Decreased complexity
Too much nitrogen can stimulate the growth of unwanted organisms, increase the biomass, and cause too rapid a fermentation. Rapid fermentation can increase aroma compound loss due to volatility, resulting in the loss of complexity. Additionally, ammonia (in the form of DAP) can prevent the appearance of aromatic degradation products from amino acids.
Amino acids are an important source of yeast esters, which can add to complexity and wine quality. Thus, the supply of nitrogen must be available to allow a continuous re-synthesis of these proteins. If that does not occur, the yeast lose the ability to conduct the fermentation.
Nitrogen addition may be effective in avoiding problem fermentations until about two-thirds of the sugar is utilized. Cells which have passed the point of transcriptional responsiveness will not respond to added nutrients. There are currently time-release nutrient products available.
It should be noted that there are significant differences between native (fruit-derived YAN) and addition products. As such, vineyard management which produces adequate fruit YAN may be very important.
Fermentation complements/addition products often contain some of the following:
- inorganic nitrogen (DAP)
- organic nitrogen (alpha-amino acids)
- unsaturated fatty acids
- sterols, thiamine, folic acid, niacin, biotin and calcium pantothenate
- magnesium sulfate
- inactive yeast cell walls
- peptides
- micro-crystalline cellulose
- other yeast autolysis products
The possible benefits of complex yeast nutrients (CYN) addition products include better resistance to oxidation as a result of increases in glutathione, higher levels of free sulfur dioxide, better color, and increased protection of aromatic quality.
I. Avoid Carbon Dioxide Toxicity.
Carbon dioxide is toxic to yeast and can impact cell performance. The release of carbon dioxide helps to minimize toxicity and decreases the lag phase of yeast growth. This is the time in which juice is most sensitive to both enzymatic and chemical oxidation.
Mixing during fermentation keeps the yeast in suspension, and helps to drive carbon dioxide out of solution, resulting in a lowering of carbon dioxide saturation. Mixing during fermentation may be important, regardless of the size and shape of the vessel. Some addition products contain inert compounds like micro-crystalline cellulose, the purpose of which is to help release carbon dioxide from solution.
More on this subject to follow.
5. French Wine Study Tour. For additional details, see Enology Notes #131.
6. New Edition of Winery Planning and Design CD Available. For additional details, see www.vtwines.info.
![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Enology-Grape Chemistry Group's web site at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes listserve send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce
Zoecklein
Professor and Enology Specialist Head Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Cell phone: 540-998-9025
Email:
