Enology Notes #161
An Alphabetical Subject Index for all past Enology Notes:
All past years' Enology Notes are still available in the Yearly Index. Use this link to jump to the most recent year's index: 2012 Enology Notes Listing.
Enology Notes #161, May 10th, 2012 - Link to PDF
To: Grape and Wine Producers
From: Bruce Zoecklein, Head, Professor Emeritus, Enology-Grape Chemistry Group, Virginia Tech
Subject:
- The 2011 Season in the Mid-Atlantic Region: A Review
- Understand the Relationships between pH and Sulfur Dioxide
- New Enology Extension Position
- Symposium on Sparkling Wine Production
1. The 2011 Season in the Mid-Atlantic Region: A Review.
Controlling the incidence of fungal degradation from Botrytis cinerea and sour rot was particularly difficult during the 2011 season in the mid-Atlantic region. While virtually every producer conducted some fruit sorting, 2011 was an excellent example of the necessity of adequate sorting, both in the field and the winery. Our goal should be to enhance our understanding of the constraints to crafting fine wines. We experienced the confluence of many of those constraints this last season, and we will undoubtedly experience them again.
As such, it is instructive to review both the wines produced last year in the context of production practices. The following is a review of fruit and wine chemistry and some management issues.
Temperature, moisture, and the presence of wounds in the fruit have a strong effect on rot development and what organisms dominate during fruit rot. Bird and insect damage were prevalent in 2011.
Botrytis cinerea is unique in its parasitology. In rainy weather, the infected grapes do not lose water, and the percentage of sugar remains nearly the same, or may decrease. Although noble rot develops regularly and uniformly, pourriture grise, or grey rot, is normally heterogeneous. Secondary infection by other microbes may follow.
Under cool and wet conditions, Penicillium, Mucor, and Aspergillus spp., as well as other fungi and yeast, may overgrow Botrytis; this is referred to in France as vulgar rot (pourriture vulgaire). Breakdown of the grape integument provides a substrate for the growth of yeasts and acetic acid bacteria, and may produce a condition called pourriture acide, or sour rot.
In contrast to the above, Botrytis infection followed by warm, sunny, windy weather causes berries to lose moisture by evaporation. With dehydration, shriveling occurs and the sugar concentration increases; this is called pourriture noble, or noble rot. Growth of the mold and associated bacteria consumes a portion of the grape sugar. However, the utilization of sugar may be countered by increases in sugar due to dehydration.
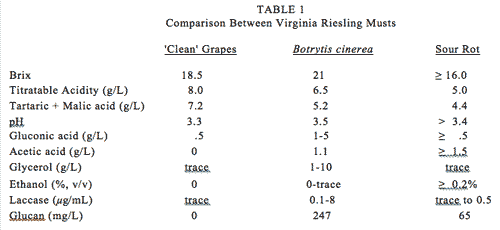
a. Effects of Fruit Rot on Fruit and Wine Chemistry. Fruit rots have significant influence on must chemistry (Table 1). The largest quantitative changes occurring as a result of Botrytis growth are those of sugars and organic acids. From 70 to 90% of the tartaric acid, and from 50 to 70% of the malic acid, can be metabolized by the mold. However, the concentration effect resulting from berry dehydration tends to obscure these effects. Change in the tartaric-to-malic acid ratio leads to a reduction in titratable acidity and elevation in fruit pH.
Botrytis and sour rot complexes use ammonia nitrogen, reducing the levels available for wine yeast metabolism. Additionally, thiamine (vitamin B1), and pyridoxine (vitamin B6) are depleted. This is a primary reason why wines produced from rot-infected grapes generally require supplementation with nitrogen and vitamins to help avoid protracted fermentations, sticking, and possible sulfur-like off odor formation. In some cases in 2011, low thiamine levels were also the result of excessive addition of sulfur dioxide to the must, binding and inactivating this important yeast growth promoter.
Like other fungi, Botrytis cinerea produces laccase, pectolytic enzymes, and esterases. Laccase catalyzes phenolic oxidation, with the resultant polymerization responsible, in part, for browning of the fruit. Perhaps of greater concern is the oxidation of aroma/flavor compounds. Laccase is resistant to sulfur dioxide, cannot easily be removed with bentonite, and is active in the presence of alcohol, including in bottled wines.
b. A Review of Rot Metabolites.
Glycerol. Glycerol is an alcohol which is produced by molds. Owing to its relatively-high specific gravity, it may contribute to the overall organoleptic perception of body when fruit glycerol levels are high: 1.04 - 1.15% vs. 0.60 - 1.1% from sound fruit. Most of the glycerol produced by molds will remain inside the defective berry despite berry dehydration, due to the fact that glycerol is non-volatile.
Botrytis-infected fruit has about 150 mg/L glycerol per one percent change in defect level by weight (Pfeffer et al., 1985). Aspergillus shows about a 300 mg/L change per one percent defect. Glycerol itself possesses no significant problem for the winemaker.
Gluconic Acid. Infected fruit can contain a relatively-high (25 g/L) gluconic acid level as a result of glucose metabolism. Since gluconic acid is not utilized by yeast or bacteria, it may be used as an indicator of fruit deterioration. Gluconic acid levels in “clean” fruit, and in wines made from clean fruit, are near 0.5 g/L, whereas in wines produced from fruit infected with B. cinerea, levels range from 1 to 5 g/L. The ratio of glycerol to gluconic acid indicates the “quality” of the rot. Higher ratios indicated the growth of true noble rot, whereas lower ratios suggest sour rot.
Acetic Acid. Acetic acid is a normal byproduct of yeast and bacteria. When acetic acid bacteria and yeast are combined with fungal growth, high levels of volatile acidity can be produced. Sour rot complex (production of acetic acid in the presence of bacteria and yeast) may show significant variations in acetic acid content in the fruit. Acetic acid is volatile at ambient vineyard temperatures and can be detected by scent during a vineyard stroll.
In some cases, fruit enters the winery showing limited visual rot, only to have excessive acetic acid produced during fermentation. Several species of Lactobacillus present in the fruit can convert grape sugars to acetic acid, thus raising the VA excessively, even prior to the completion of alcoholic fermentation.
Ethyl Acetate. The volatile character or “acetic nose” is not exclusively the result of acetic acid production. Acetate esters, most specifically ethyl acetate, contribute significantly to this defect.
Ethyl acetate formation by yeast occurs by chemical esterification, as illustrated below. Ethyl acetate produced by lactic acid bacteria is the result of sugar metabolism, hence the reason why the VA may increase significantly during fermentation.
![]()
Galacturonic Acid. Botrytis causes an increase in the galacturonic acid content as a result of enzymatic hydrolysis of cell wall pectin compounds. Galacturonic acid may be transformed to mucic (galactaric) acid by enzymatic oxidation, and may reach must levels as high as 2 g/L. This acid can combine with calcium to form an insoluble salt, calcium mucate. This can be a large potential problem if the winery water source has a high (> 40 mg/L) calcium level (for additional information, see Calcium Mucate in the Enology Notes Index at www.vtwines.info).
c. Polysaccharides and Instability. One of the greatest impacts of fruit rot is the formation of polysaccharides that create clarification problems. Polysaccharides can form protective colloids in juices and wines, inhibiting clarification. Pectins (complex sugars that hold plant tissues together) are hydrolyzed by mold-produced enzymes, with the formation of soluble pectin and beta-1,2- and -1,6-glucans.
In wine, ethyl alcohol causes the pectins and glucan chains to aggregate, thus inhibiting clarification and filtration. Pectinolytic enzymes and glucanase enzymes are available to minimize these clarification problems. Zoecklein et al. (1995, 2005) provides two simple lab procedures for determining pectin and glucan instability. It is highly recommended that wines be evaluated for filterability and/or pectins/glucan prior to filter set-up.
d. Aroma and Varietal Character. In 2011, in the mid-Atlantic region, general environmental constraints (limited fruit maturity, uneven maturity) in part contributed to limited varietal character. Additionally, aroma compounds can be lost as a result of the oxidizing effect of fruit rots. Metabolites such as gluconic acid, oxidase enzymes, volatile esters, aldehydes, and traces of other organic compounds may alter grape aroma/flavor compounds or their aroma intensities.
Post-fermentation addition of pectinolytic enzymes may increase in grape-derived aroma intensity. Most pectinolytic enzymes contain glycosidases which can convert grape aroma/flavor precursors to their odor-active forms. Additional information is available in the Enology Notes Index at www.vtwines.info.
Muté Production. Mutés (juice held, or mutéd, from fermentation) can add life and freshness back into the base wine. A small quantity of muté produced from non-degraded fruit can help recover lost aroma while masking some of the acetic and oxidized tones which may have resulted from sour bunch rot. Specifics regarding muté production are discussed in Controlling Microbial Growth in Wine, an on-line publication available at www.vtwines.info.
e. Future Production Considerations. The following are a few additional considerations to keep in mind for upcoming seasons if the threat of fruit rot is high. Details regarding each item listed are provided in previous Enology Notes found at www.vtwines.info. Also see the slide show on my web site authored by Dominique Delteil titled: Good Practices for Gray Rot Affected Grapes
- Crop level: Avoid over-cropping which could delay maturity.
- Fruit culling: Cull as much fruit rot out as possible in the field.
- Fruit sorting: Sort fruit at the winery. A very small concentration of rot can have a large impact. It is not the incidence of rot, but the level of various rot metabolites that determine how much rot is acceptable. The best rule of thumb: no rot is acceptable.
- Rinse fruit: You may consider rinsing the fruit with water if the fruit delivered to the winery is high in rot. That will help to lower some of the sour rot metabolites and late season spray residues. Not practical for large volumes this practice can slightly lower the Brix level as a result of dilution.
- Muté production/cryoextraction: A small quantity of muté produced from non-degraded fruit can help recover lost aroma and aroma intensity resulting from sour bunch rot.
- Dehydration. Only extremely ‘clean’ fruit should be used for this style of wine production.
- Pressing: Whole cluster press whites by discarding the initial juice. Press very lightly and take press fractions.
- pH adjustment: Adjust the juice pH. The lower, the better. Expect about 2.0 g/L TA will drop out during fermentation or shortly following completion. Last season, it was the high pH values that caused many to have both biological and oxidative problems.
- Sulfur dioxide: Keep the initial sulfur dioxide level low during pressing. You want the low molecular weight tannins to polymerize or bind together. Then raise the sulfur dioxide, depending on the fruit condition and pH.
- Cold settle. Adequate cold settling with the use of pectinolytic enzymes will help lower the level of rot metabolities.
- Tannin addition. You could add enological tannin. That would help clarify the juice and bind with some of the rot-produced enzymes. Tannins can act as oxygen buffers and may bind with enough protein to lower the bentonite requirement needed for wine protein stabilization. This is an important consideration for rather delicate varieties such as Pinot gris and Sauvignon blanc.
- Pectinolytic enzymes: The addition of pectic enzymes aids in clarification, which is particularly important if juice is produced from compromised fruit.
- PVPP: Add some PVPP inline to the juice if there is a high level of grape tissue degradation.
- Ascorbic acid: For varieties where the oxidation potential is large, add ascorbic acid to the juice.
- Test YAN: Test the YAN content and make adjustments accordingly. Rots deplete YAN. Note that rots also lower the micronutrient levels. As such, the addition of a complex nutrient formulation, not simply DAP, is wise.
- Measure the NTUs: You want to ferment fairly-clean juice. Measure the NTUs if you can. If you measure, you will want about 100 to 150. Again, if the juice is not clarifying, you may want to add enzymes or more tannin. Don’t add them together.
- Inoculation: Inoculate with a high volume of a vigorous, not too N-dependent yeast. Use more than the standard 24 g/hL or 2 lb/1000 gallons. Make sure the starter is properly prepared, and understand that oxygen is a yeast nutrient.
- Co-ferment: If you are planning on an MLF co-fermentation, make sure you check with your suppliers regarding yeast and MLF strain compatibility. If you do not desire an MLF, consider the use of lysozyme.
- Fermentation temperature: Begin the fermentation at a slightly warmer temperature to help lower the concentration of undesirable aroma characters, and to assure a rapid yeast fermentation.
- Mid-fermentation racking: Rack mid fermentation. This helps to remove wine from the primary lees.
- Rack immediately post-fermentation.
- Consider short vatting reds, and avoid extended post-fermentation maceration. Use short vatting, and possibly délestage, to help remove fermenting wine from lees. Ferment in the presence of non-toasted wood and carefully review the steps listed above.
2. Understand the Relationships between pH and Sulfur Dioxide.
Most winemakers are fully aware of the relationship between pH and molecular free sulfur dioxide. It should be noted that acetic acid bacteria in red wines are not inhibited by pigment-bound sulfur dioxide. Because of the rapid change in equilibrium, standard analyses report this sulfur dioxide as free. This is false, due to the fact that this is rapidly consumed by phenols. This explains, in part, how we can have the production of high VA in the presence of seemingly-adequate sulfur dioxide.
3. New Enology Extension Position.
Many of you received this notice directly in a previous communication. Our Department of Food Science and Technology at Virginia Tech is looking for an Enology Extension Specialist.
As many are aware, I retired from my position at Virginia Tech in July 2010. At that time I was re-hired in a 1/3 time appointment. Throughout my tenure at Virginia Tech I had a three-way appointment split: teaching, research, and extension, plus I established and oversaw the Enology Service Laboratory. The University realized (all be it after the fact) that my position should be split into several.
This job is new and is a 100% extension, full-time (12 month) non-tenure track appointment. The primary responsibility will be to continue to expand the educational resources available to Virginia wine producers.
The successful candidate will support the continued growth and development of Virginia’s wine industry through the expansion of educational extension programs and applied enological research; provide written and oral educational resources to existing and potential wine industry practitioners; and provide an educational bridge between vineyard practices and the wine production practices that directly impact wine quality.
MS in food science or related discipline is required with education and/or experience in best management practices for enology and viticulture. Preference will be given to candidates with effective communication skills and ability to work within a team environment.
More information is through my office or available at https://listings.jobs.vt.edu/applicants/Central?quickFind=195354.
4. Symposium on Sparkling Wine Production
The American Society for Enology and Viticulture eastern section 37th annual conference and sparkling wine symposium will be held on Thursday, July 16-19, 2012, in Traverse City, Michigan. Details can be found at http://asev-es.org/.
Additional information on Méthode Champenoise sparkling wine production can be found at www.vtwines.info (click On-Line Publications).
References
Pfeffer, T.E., C.D. Clary, and V.E. Petrucci. 1985. Adaptation of HPLC to wine grape inspection. Report to the Wine Grape Inspection Advisory Committee, CDFA, Viticulture Research Center, California State University, Fresno.
Zoecklein, B.W., K.C. Fugelsang, B.H. Gump, and F.S. Nury. 1995, 2005. Wine analysis and production. 621 pp. Kluwer Academic/Plendum Plublishers, New York, NY.
![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Enology-Grape Chemistry Group's web site at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes listserve send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce
Zoecklein
Professor and Enology Specialist Head Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Cell phone: 540-998-9025
Email:


