Enology Notes #160
An Alphabetical Subject Index for all past Enology Notes:
All past years' Enology Notes are still available in the Yearly Index. Use this link to jump to the most recent year's index: 2011 Enology Notes Listing.
Enology Notes #160, September 3rd, 2011 - Link to PDF
To: Grape and Wine Producers
From: Bruce Zoecklein, Head, Professor Emeritus, Enology-Grape Chemistry Group, Virginia Tech
Subjects:
1. Phenols, Wine Quality, and Reductive Strength.
The following is a review of some of the issues discussed during our pre-harvest short courses. The phenolic compounds in wines are the result of a host of factors, including those depicted in Figure 1.
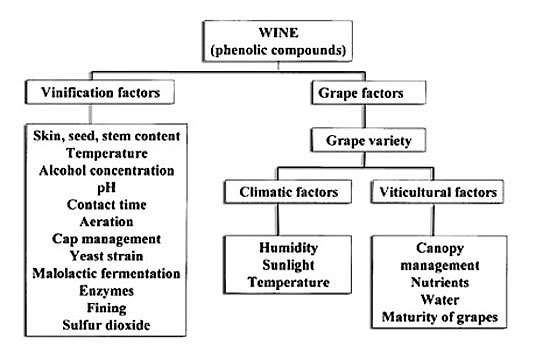 Figure 1. Factors affecting phenolic compounds in wines.
Figure 1. Factors affecting phenolic compounds in wines.
(In part, from Stockley and Høj, 2005.)
Approximately one-third of the carbon produced by grapevines is used to make phenolic substances. Therefore, phenols are an important constituent of both grapes and wines.
It is the structure of a wine, not the composition that determines flavor and integration. The integration of the structural and textural elements, and has been discussed in previous editions (see Enology Notes #52, 68, 69, 76, 84, 107, 108, and 113).
Kassas and Kennedy (2011) noted some interesting positive correlations in red wines. In their study, wines commanding the highest price in the marketplace had several attributes:
- highest concentrations of total tannins
- highest concentration of skin tannins
- degree of phenol polymerization
The term tannin defines a very heterogeneous group of phenolic compounds that are identified, based on certain properties:
- astringency
- bitterness
- reaction with ferric chloride
- ability to bind with proteins, e.g., tannin leather – hence, the term tannins
It was their characteristic interaction with proteins that traditionally differentiated tannins from other phenols. However, not all phenols that bind with proteins elicit an astringent response, and tannins are not the only compounds in wines that cause astringency.
Some phenols, including tannins, have the ability to polymerize, or associate, with themselves and other compounds, including anthocyanin pigments. As polymerization occurs, the molecule becomes larger. The number of subunits bound together is referred to as the DP number, or degree of polymerization. So-called tannin “quality” relates to the following:
- degree of polymerization
- association of tannins with other molecules
- stereospecific nature of the molecule, which can make it harsh and hard, or supple
Grape tannins derived from the skin, seeds, and stems differ in their sensory properties, and their DP number and overall subunit concentration. Tannin perception is a function of the factors shown in Figure 2.
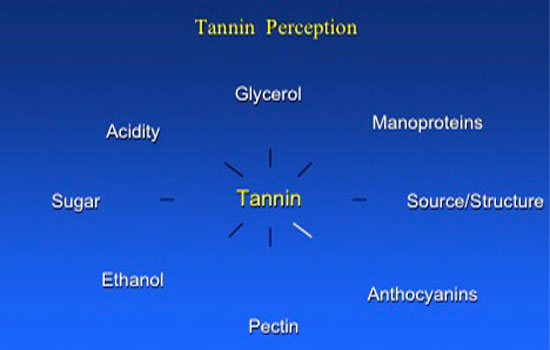
Figure 2. Factors influencing tannin perception.
a. Pigment-Tannin Polymerization. In grapes and wines, anthocyanin pigments can be either free monomers, that is, unbound, or associated with other phenols to form polymers.
Anthocyanin-tannin polymerization occurs both, in the fruit during maturation, and in wines. Polymerization continues until an anthocyanin molecule binds the terminal end of the tannin chain, thus stopping the polymerization (see Figure 3).
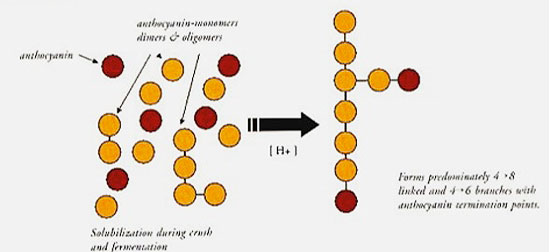 Figure 3. Proposed condensation reactions under non-oxidative conditions.
Figure 3. Proposed condensation reactions under non-oxidative conditions.
(Source: McCord, 1990.)
As such, the ratio of anthocyanins to tannins is important in impacting the extent of polymerization. This is highly crucial, because the degree of polymerization affects astringency.
Large tannin polymers provide a relatively large number of binding sites to interact with proteins, including salivary proteins. Wines with an abundance of large polymers tend to lack softness, may lack color stability, and often possess a dry mouth sensation.
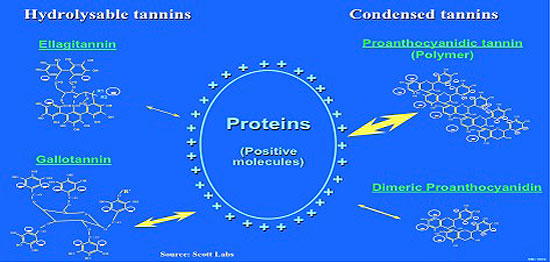 Figure 4. Reactivity of tannins. (Source: Scott Labs.)
Figure 4. Reactivity of tannins. (Source: Scott Labs.)
Smaller polymers, on the other hand, have fewer protein binding sites. As such, they produce less astringency, and provide a greater degree of soft tannins and more palate depth. Additionally, these smaller pigment polymers provide a greater reductive strength (see below).
The more anthocyanins, the shorter the resulting polymers and the finer the tannins. Smaller polymers lead to smaller colloids which have a softer mouthfeel (Smith 2010).
Binding with phenols not only can involve other phenols like anthocyanins but also hydrogen sulfide, mercaptans and sulfur containing amino acids (Smith 2011).
b. Reductive Strength. Longevity, or the ability to age, is an important quality attribute. The reductive strength of a wine is a measure of the uptake of oxygen. This is influenced by the phenol composition and lees, among other things.
Some phenols, including tannins, have the ability to react with oxygen, bind with another phenol and recreate the original structure-thus allowing it to react over and over again.
Remarkably, the reaction of a young wine with oxygen can make that wine more resistant to later oxidation. This means young wines can consume oxygen and this actually increases reductive strength.
As discussed by Smith (2010), Randall Grahm of Bonny Doon Vineyards considers reductive strength to be analogous to a wine’s chi or, as the Chinese would say, life force. When a wine is young, it can share its chi with the world; when old, it must guard it so the wine does not diminish too quickly. Young wines have a capacity to adsorb oxygen and that can actually increase its resistance to later oxidation. Irrespective of chi, we believe that reductive strength is related to the phenolic composition of a wine and, therefore, to longevity.
Clark Smith reviewed the impacts of fruit maturity on reductive strength at the 2011 Wineries Unlimited meeting in Richmond. He suggested that the problems with under-ripe fruit include the following:
- Insufficient pigments
- Limited extraction
- Limited desirable flavors, which limits tannin capacity
The problems with over-ripe fruit include the following:
- Loss of color
- High alcohol capacity, which can destabilize color
- Significant loss of reductive strength in the resultant wine
The change in the phenolic content, as a function of excessive fruit maturity, can lower the reductive strength by a factor of 10 (Smith, 2011). The following (Figure 5) illustrates the variation that can occur between the skin tannins and anthocyanin concentration among seasons. While seed tannins are less affected, note the large potential difference in the ratio of tannins to anthocyanins.
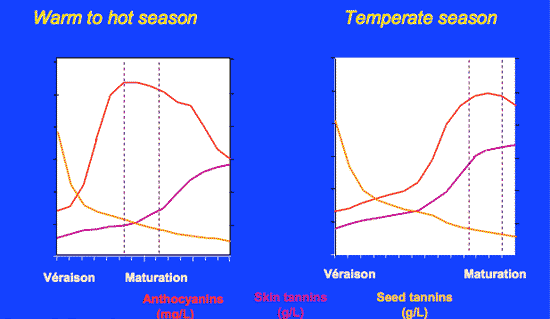 Figure 5. Effect of climate on relative phenolic concentration. (Source: Lallemande.)
Figure 5. Effect of climate on relative phenolic concentration. (Source: Lallemande.)
Grapes producing the most intense, balanced wines with the greatest longevity usually have a high anthocyanin to tannin ratio. Indeed, wine quality may be dependent, in part, on this ratio (see Enology Notes #8, 103).
Some changes that can be measured during fruit maturity to evaluate phenolic development include the following (Figure 6):
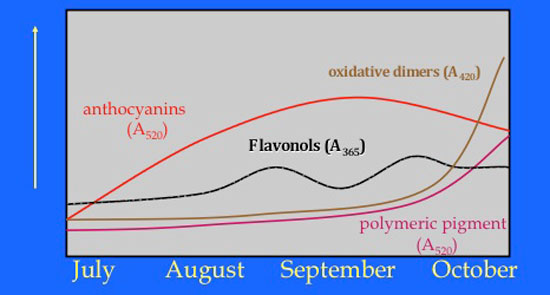 Figure 6. Wine grape phenolic maturity. (Source: Smith, 2011.)
Figure 6. Wine grape phenolic maturity. (Source: Smith, 2011.)
Oxidative dimers refer to tannin-tannin polymers, and polymeric pigments to the tannin-anthocyanin complexes discussed above. Flavonol phenols are essentially the grape’s sunscreen. They are produced as a function of sun exposure. Their concentration can be increased by a factor of 8 in sun vs. shaded fruit. The Enology Service Lab at Virginia Tech is conducting these analyses for the industry. The main advantage is to allow for an understanding of the free or unbound anthocyanin content vs. those bound to tannins. Call my office for more information, or see www.vtwines.info.
Under the leadership of my colleague Clark Smith we are conducting several studies evaluations of reductive strength. We hope to have some practical information to share.
c. Factors Impacting Red Wine Color. Color is an important wine attribute, because humans are visually oriented. As such, wine color can certainly bias evaluations. A classic example of color bias is to change the color of a white wine, such as Chardonnay, with red food coloring. In blind evaluations, the color-adjusted wine frequently receives a different sensory rating for attributes such as fullness, body, and complexity.
As such, richly-colored wines are assumed to have high volume or body, and softer tannins. Conversely, a wine with less color is automatically assumed to have “green” or “harsh” tannins.
Spectral color in wine is a function of these three elements:
- anthocyanin concentration
- polymeric pigments
- concentration of cofactors, or certain non-colored compounds, which bind with anthocyanins
Hyperchromicity, also known as copigmentation, is an interesting phenomenon that allows more visible red color than would be expected due to the anthocyanin concentration alone. Cofactors are non-colored compounds that have the ability to bind with anthocyanins, creating more color than the unbound pigment, hence the term hyperchromicity.
The concentration and type of cofactors vary greatly from variety to variety, season to season, but include some non-flavonoid phenols, flavonols, and the amino acid arginine. It is not likely that enological tannins contain compounds that act as cofactors.
Because red color is a function of three elements (anthocyanin concentration, cofactor concentration, and polymeric pigments), it is possible to have the following (Boulton, 2005).
- Change in grape anthocyanin concentration = Change in wine color
- Change in grape anthocyanin ≠ Change in wine color
- No change in grape anthocyanin = Change in wine color
The above highlights several points:
- Variation in cofactors and polymeric pigment concentration may be more important to spectral color than simple anthocyanin concentration.
- Grape pricing based on anthocyanin concentration alone may not be desirable.
- Harvesting based on anthocyanin concentration will not necessarily assure desirable red wine color.
Young wines have a high concentration of unbound pigments. Once they are incorporated into polymers with tannins they are stabilized. Early stabilization via oxygen exposure is a significant benefit to both color, texture and aromatic properties. Because much of the phenol polymerization is oxidative anything that depletes the oxygen content early may be deferential. As discussed by Smith (2010) lees contact can lower the oxygen content and therefore may reduce incorporation of anthocyanins into stable pigment polymers.
2. Pre-Harvest and Harvest YAN Analysis. Again this season Virginia Tech’s Enology Service Laboratory will conduct YAN and YAN component analyses for the industry.
- Email the Laboratory at to request processing bags and bottles
- Include name, company, mailing address, and the number of sampling kits required
- Collect juice samples at harvest, or grape berry samples from the vineyard pre-harvest*
- Fill sample bottles to the indicated level, mix thoroughly to dissolve preservative
- Ship samples overnight to the Laboratory; shipping delays impact analysis results
*Complete information regarding sampling and berry-bag processing can be found on the VT Enology-Grape Chemistry Group website (www.vtwines.info) under Online Publications > Maturity Evaluation for Growers.
The results are strongly dependent on adequate and representative sampling in the vineyard and proper sample processing.
Ship samples to:
Wine/Enology-Grape Chemistry Group
Enology Service Lab
Attn: Ken Hurley
Rm. 113, FST Bldg.
Virginia Tech (0418)
Blacksburg, VA 24061
For more information on available analyses and analytical panels, including prices, please see the Enology-Grape Chemistry website at www.vtwines.info.
References
Boulton, R. Verbal communication.
Kassara, S. and J.A. Kennedy. 2011. Relationship between red wine grade and tannin composition. 2. Tannin composition and size. J. Agric. Food Cem. 59: 8409-8412.
Smith, C. 2010. Red wine structure. Goal and strategies. Presentation of the 2010 Wineries Unlimited, Richmond, VA
Smith, C. 2010. Probing wine’s anti-oxidant potential. Practical Winery and Vineyard, Jan/Feb 53-57.
Smith, C. 2011. Phenol chemistry and winemaking. Wines and Vines. April 54-59.
Stockey, C. S., and P.B. Hoj, 205. Better wine for better health: Fact or fiction? Aust. J. Grape Wine Res. 11:127-138.
![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Enology-Grape Chemistry Group's web site at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes listserve send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce
Zoecklein
Professor and Enology Specialist Head Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Cell phone: 540-998-9025
Email:


