Enology Notes #156
All past years' Enology Notes are still available. Use this link to jump to the most recent year's index: 2010 Enology Notes Listing.
Enology Notes #156, November 19th, 2010 - Link to PDF
To: Grape and Wine Producers
From: Bruce Zoecklein, Head, Enology-Grape Chemistry Group, Virginia Tech
Subjects:
- Dr. Bruce Zoecklein Awarded Professor Emeritus Status by the Virginia Tech Board of Visitors
- Wine Tannins
- Volatile Acidity
- Controlling Microbial Growth in Wine
- Winery Planning and Design, Edition 16, Available
1. Dr. Bruce Zoecklein Retires and is Awarded Professor Emeritus Status by the Virginia Tech Board of Visitors. In July, I retired as Professor and Head of the Enology-Grape Chemistry Group at Virginia Tech. Subsequently I was awarded emeritus status by the Virginia Tech Board of Visitors.
I have been fortunate to be a faculty member at Virginia Tech since 1985, truly one of the most outstanding academic institutions in the USA. My contributions to the teaching, research, and extension missions have been recognized by the University and my personal academic goals realized. While I wanted time to pursue other interests, I will continue to work for Virginia Tech in a limited research and extension capacity.
This Enology Notes series will continue, and the Enology–Grape Chemistry Group website will be maintained. Additionally, I will continue to oversee the Enology Service Lab that I established in 2006 at Virginia Tech.
Our challenges in crafting fine wines include the understanding of the following relationships:
- Environmental factors, vineyard management, and fruit chemistry
- Fruit chemistry and wine chemistry
- Wine chemistry and sensory properties
To date, more than 1000 compounds have been identified in grapes and wines. The next great advancements will come in our understanding of the effects of individual compounds on wine. We also need to learn how to manage the chemical and physical properties that, in combination, shape a wine’s sensory profile.
We have a number of sensory descriptors used for tannin impression, including silk, velvet, melted, hard, and green. These represent qualitative and quantitative differences in wines. For example, some tannins appear to be more astringent at higher concentrations, but more bitter at lower concentrations (Jackson 2009). Complex, polymerized tannins (those that have been bound together) tend to be astringent, while tannin monomers tend to be primarily bitter, and moderate-size tannins often create the perception of both bitterness and astringency.
What are the features that impact the sensory impressions of tannins?
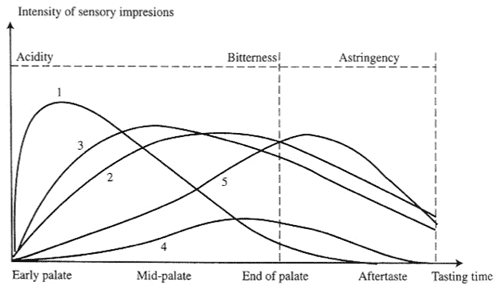
Figure 1. Impact of flavonoid polymerization on sensory attributes.
(Jackson 2002 adapted from Glories 1981)
Glories (1981) demonstrated the difficulty in distinguishing astringency impact from the sensory influences of acidity and bitterness. Figure 1 demonstrates the impact of flavonoid polymerization on sensory attributes. The figure illustrates the sensory response from simple flavonoids (curve 1), and increased polymerization, curves 2 and 3. The sensory response to anthocyanins is illustrated in curve 4, and to stem tannins in curve 5. Figure 1 highlights the difficulty in separating the sensory impact of acidity, bitterness and astringency.
a. Tannin interaction with proteins and polysaccharides. The formation of tannin colloids may contribute to the softening or reduction in wine astringency. Young red wines contain monomeric anthocyanins and unpolymerized tannins. These insoluble compounds gather together to form co-pigmented colloids, as seen in Figure 2.
Colloids are important for tannin ‘age’ and mouthfeel (Kennedy 2010).
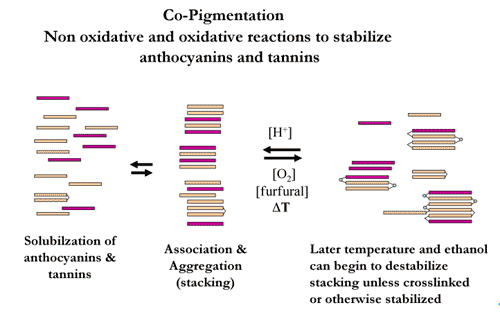
Figure 2. Co-pigmentation (McCord, 2002)
Salivary proteins can bind onto the surface of co-pigmented colloids, articularly those containing monomeric anthocyanins and tannins, resulting in a perception of green or grainy-type tannins. These are generally noted in the front of the palate and are often confused with acidity (Smith 2010).
Monomeric (single-unit) tannins can undergo non-oxidative and oxidative polymerization or binding (Figure 2). Both types of reactions are very important to help attain color stability and optimum mouthfeel.
As discussed in previous editions, the ratio of anthocyanins to tannins is very important in the impact on binding. Polymerization stops when an anthocyanin molecule is attached to a tannin. Oxidative polymerization creates acetaldehyde (from the oxidation of ethyl alcohol, such as occurs with splash racking and microoxygenation) to net together anthocyanins and tannins (Figure 3). As can be seen, polymer formation is different for oxidative and non-oxidative reactions. The binding sites differ but more importantly the geometry of the molecules can be different, resulting in differences in availability of reactive sites to bind with salivary proteins.
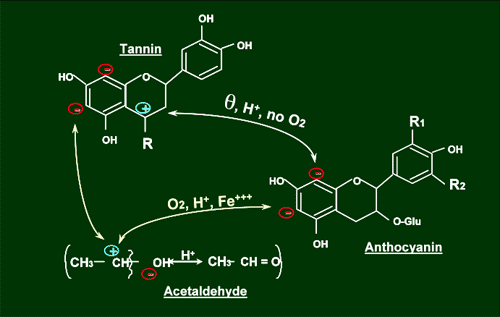
Figure 3. Production of acetaldehyde through oxidative polymerization.
Non-oxidative polymerization produces polymers that are compact. As such, they have limited protein-binding ability, because their reactive groups are not well exposed. Therefore, they have a limited impact on mouthfeel.
As polymerization continues, the tannin chain length increases. This occurs with age and with continued microoxygenation. The saliva-protein binding is increased, and dry or even dusty-types of tannin perception can be created.
As this occurs, the co-pigmented polymer becomes increasingly insoluble.
As stated, oxygen in young red wines helps to create oxidative polymers by forming acetaldehyde, which creates the ‘bridge’ by which tannins and anthocyanins can bind (Figure 3) .
Oxygen helps to increase the chain length by allowing the binding of tannins and anthocyanins. These co-pigmented polymers are rather open, allowing for significant binding with saliva proteins, creating a strong impression of astringency (Smith 2010).
Excessive openness of these co-pigmented colloids can result in excessive astringency. The co-pigment–saliva protein interaction, and thus the perception of tannin astringency, can be modified by incorporation of lees peptides, etc., into these polymer chains.
Winemakers are making use of extended aging of wine on secondary lees. In the Burgundy region, red wines are aged on lees in conjunction with the addition of exogenous β 1,3-glucanase enzyme. This procedure is an attempt to increase release of mannoproteins, which may enhance suppleness by reducing the perceived astringency of tannins (See Enology Notes Index at www.vtwines.info for additional information).
Mannoproteins found in the yeast cell wall are bound to glucans, or glucose polymers. Wine mannoproteins exist as polysaccharides and proteins. They are released from the yeast cell wall by the action of an enzyme, β 1,3-glucanase, upon the wall.
Thus, tannins can associate with other large molecules, such as polysaccharides and mannoproteins, which significantly impacts their saliva protein-binding ability and, therefore, their sensory attributes. The stability of these colloids may be an important feature. It has been suggested that colloid disruption may help to explain bottle shock (Kennedy 2010).
The interaction between tannins and proteins becomes weaker as tannins age. McRae et al. (2010) demonstrated that grape tannins have a much stronger interaction with proteins than do a 10-year-old wine’s tannins. As suggested by Kennedy et al. (2010), the next generation of tannin analyses to help quantify mouthfeel may rely on tannin protein-binding strength, rather than simply tannin concentration.
Studies using the gelatin index technique to evaluate the degree of astringency have shown that wine tannins are less astringent when in the presence of mannoproteins.
For example, a control wine, whose gelatin index is 68%, shows an average index of 34.6% when fermentation derived mannoproteins are added, and an average index of 26.4% when autolysis derived mannoproteins are added, reflecting a decrease in astringency of the wine and illustrating the effects of fermentation versus autolysis-derived mannoproteins.
There is a strong relationship between wine tannins and wine aroma. Sometimes sulfur-containing compounds are incorporated into co-pigment–colloid chains. This may explain the lowering of the perception of sulfur-like off odor in wines which have undergone some microoxygenation. Additionally, Saenz-Navajas et al. (2010) have demonstrated the impact of aroma on mouthfeel. They provide evidence that fruit aroma can influence the perception of sweetness, and thus reduce the perception of astringency.
- Astringency is impacted by the stereo-specific nature, number of hydroxyl groups, the way these bind with saliva, saliva flow, pH, viscosity, sweet taste, and non-soluble solids
- Lower pH – higher astringency
- Higher alcohol – higher bitterness
- Incorporation of anthocyanins terminates tannin polymerization
- Generally, greater color = finer tannins
- Interaction of salivary proteins can be blocked by incorporation of lees peptides and other sulfur-containing side groups
- Increased polymerization augments drying, chalky, grainy, puckery attributes
The following is adapted from Zoecklein et al (2005). The total acidity of a wine is the result of the contribution of nonvolatile or fixed acids, such as malic and tartaric, plus those acids separated by steam volatilization, or volatile acids. A measure of volatile acidity is used routinely as an indicator of wine spoilage.
Although generally interpreted as acetic acid content (in g/L), a traditional volatile acidity analysis includes all those steam-distillable acids present in the wine. Thus, significant contributions to volatile acidity (by steam distribution) may be made by carbon dioxide (as carbonic acid), sulfur dioxide (as sulfurous acid) and, to a lesser extent, other organic acids.
a. Microbiological Formation of Acetic Acid. The volatile acidity of a sound, newly-fermented dry table wine may range from 0.2 to 0.4 g/L. Increases beyond this level, however, may signal microbial involvement and potential spoilage. The principal source of acetic acid post-fermentation in stored wines is attributed to growth of acetic acid bacteria and certain lactic acid bacterial species.
b. Formation of VA by Spoilage Yeasts. In some cases, high levels of volatile acidity may result from growth of yeast during fermentation. There is considerable variation in production of acetic acid and other byproducts among both native and cultured wine yeast strains of Saccharomyces spp.
Among those yeasts involved in acetification of wine, Brettanomyces is known to produce relatively large amounts. In one study, acetic acid production by Brettanomyces in white wine after 26 days of incubation (28°C/82.5°F) increased from 0.31 g/L to 0.75 g/L.
Acetic acid is a normal by-product of yeast growth and has its origin primarily in the early stages of fermentation. Several intrinsic and extrinsic factors may affect formation of acetic acid by yeast, including the following:
- pH
- Sugar
- Available nitrogen
- Fermentation temperatures
- Interactive effects of other microorganisms
- Botrytis and other fruit fungi
pH impacts acetic acid production, with more acetic acid produced at low (<3.2) pH.
The effect of increased osmotic pressure, resulting from high-sugar musts, on volatile acid formation is well known. Such fermentations typically have a longer lag phase with reduced cell viability and vigor. Generation time (budding) is also delayed. At initial fermentable sugar levels above 20%, acetic acid increases with sugar level and has been found to range from 0.6 to 1.0 g/L in musts of 32 to 42°Brix (17.7 to 23.3°Baumé), compared with controls at 22°Brix (12.2°Baumé) with acetic acid of 0.4 g/L. Visually, yeast cells growing under conditions of high osmotic pressure appear stressed.
Must nitrogen levels may also play a role in acetic acid formation. When available nitrogen is low, higher initial sugar levels (as seen in over-ripe or mold-damaged fruit) may lead to increased production of acetic acid.
Fermentation temperature is also known to affect the levels of acetic acid produced by wine yeasts. An early study found that volatile acid formation increased with increased fermentation temperature, over the range of 15°C (59°F) to 25°C (77°F).
Significant differences between yeast strains have been reported. In one study it was noted that with two strains of S. cerevisiae the formation of acetic acid was maximal at 40°C (104°F) in one case, whereas maximum formation occurred at 10°C (50°F) in the second strain.
Unless controlled, the temperature of fermentation may rise to a point at which it becomes inhibitory to wine yeast. In practice, inhibition may be noted at temperatures approaching 35°C (95°F) or higher. Because acetic and lactic acid bacteria can tolerate temperatures higher than those needed to kill (inhibit) wine yeasts, stuck or protracted fermentations often are susceptible to secondary growth of these organisms.
Pressure fermentations may also result in higher than expected volatile acid content, possibly due to selective inhibition of wine yeasts and growth of lactic acid bacteria.
c. Post-Fermentation Sources of Volatile Acidity. Cellar practices play an important role in volatile acid formation in stored wines. High levels of VA may result when headspace (ullage) is allowed to develop. In this case, the combination of oxidative conditions and surface area may support rapid growth of both bacteria and yeast. Because acetic acid bacteria are aerobic (air requiring) organisms, depriving them of oxygen is a viable means of controlling further growth. However, controlling growth requires a significant reduction in oxygen (to about ½ percent). Wood cooperage does not provide the complete airtight (anaerobic) environment needed to completely inhibit growth of air-requiring organisms.
Acetic acid bacteria may survive and grow at low oxygen levels present even in properly stored wines. Viable populations of Acetobacter present in properly maintained wines in wood cooperage can survive in low numbers. The bacteria can survive due to slow exchange of oxygen (approximately 30 mg/L/year) into the wine. Transitory exposure to air, such as may occur during fining and/or racking operations, etc., may be sufficient to stimulate growth. Although the exposure may be short term and the wine is subsequently stored properly, incorporation of oxygen can support continued growth of the bacterium. The problem becomes more apparent with increases in cellar temperature and wine pH.
During proper barrel storage, a partial vacuum develops within the barrel over time. Both water and ethanol diffuse into the wood and escape to the outside as vapor. In cellars where the relative humidity is less than 60%, water is lost from the wine to the outside environment, and the alcohol content of the wine increases. Conversely, where a higher relative humidity exists, alcohol is lost to the outside environment. Diffusion of water and ethanol through pores in the staves creates a vacuum in the properly-bunged barrel. Thus, even though some headspace may develop under these conditions, the oxygen concentration is very low. Formation of a partial vacuum in the headspace requires tightly-fitted bungs. Topping sealed barrels too frequently results in loss of vacuum and may accelerate both oxidation and biological degradation of the wine.
The volatile acidity of properly maintained barrel-aged red wines may increase slightly without the activity of microorganisms. An increase in volatile acidity of 0.06-0.12 g/L as acetic acid is inevitable after one year in new wood, not as a result of biological degradation, but due to hydrolysis of acetyl groups in the wood hemicellulose, and the result of coupled oxidation of some wine phenolics.
Although the practice is not recommended, winemakers forced to store wines in partially filled containers often blanket the wine with nitrogen and/or carbon dioxide. Nitrogen is the preferred blanketing gas, because of its limited solubility in wine. Sparging of wines (introduction of micron-size bubbles) with carbon dioxide is a better practice, allowing the gas to dissolve in the wine. Upon standing, the gas escapes slowly from solution and, due to its density, remains at the wine’s surface to offer a degree of protection against oxidative deterioration and partially controlling air-requiring microorganisms.
d. Acetate Esters. The volatile character or “acetic nose” is not exclusively the result of acetic acid. Acetate esters, most specifically ethyl acetate, contribute significantly to this defect, providing an odor of nail polish remover.
Factors that can influence formation of acetate esters include yeast strain (as well as presence and population density of native yeasts), temperature of fermentation, and sulfur dioxide levels.
The growth of Hanseniaspora uvarum and Kloeckera apiculata yeasts during the early phase of fermentation results in significant production of ethyl acetate. These species frequently represent the dominant native yeast flora, and their numbers may increase significantly, even in fermentations inoculated with active Saccharomyces starters. Other native yeast species are known to produce substantial amounts of ethyl acetate (and other spoilage esters).
e. Ethyl Acetate and Spoilage. Although high acetic acid content and the presence of ethyl acetate are generally associated with each other, they may not always be produced to the same extent. Ethyl acetate levels of 150 to 200 mg/L impart spoilage character to the wine. It has been suggested that a maximum ethyl acetate level of 220 mg/L be used, rather than traditional analyses of acetic acid as an indicator of spoilage. This suggestion is based on the fact that high acetic acid content does not always confer spoilage to the wine. A volatile acid content of less than 0.70 g/L seldom imparts spoilage character and, in combination with low concentrations of ethyl acetate, may contribute to overall wine complexity.
Acetic acid and ethyl acetate levels in unfermented must have also been examined as indicators of spoilage in grapes.
f. Sensory Considerations. Volatile acidity magnifies the taste of fixed acids and tannins but, itself, may be somewhat masked by high levels of sugar and alcohol. This may help explain why VA can be sensorially detected in some wines at relatively low levels (<0.5 g/L), whereas in others it is not noticeable at even higher concentrations.
g. Reduction of Volatile Acidity. Both TTB and the OIV regulate the levels of volatile acidity (expressed as acetic acid) in domestic wines offered for sale. In California, more restrictive regulations apply.
Reduction of high volatile acidity in wines is difficult. Attempts to lower volatile acid levels by neutralization generally yield undesirable results, because of concomitant reduction in the fixed acid content. Similar problems (flavor and aroma stripping and modification) are encountered in the use of ion exchange. Reverse osmosis has proven successful. Use of yeast for volatile acid reduction has also been studied; the application takes advantage of oxidatively-growing yeasts using acetic acid as a carbon source. Utilization of acetic acid by active yeasts has led some winemakers to add high volatile acid wine to fermenting musts to lower volatile acid levels. However, such practices run the risk of contaminating the entire lot, and may have a detrimental impact on fermentation, as well as on final wine quality. Judicious blending is probably the best practice to use in lowering the volatile acid content of borderline wines.
4. Controlling Microbial Growth in Wine.
There are a number of steps that can be used to help control microbial growth in wine which, collectively, can be effective. Each of the features below has been outlined in editions of Enology Notes, available at www.vtwines.info. Click “Enology Notes” and “Enology Notes Index”:
- Proper sanitation
- Proper sanitation monitoring
- Lysozyme
- Sulfur dioxide
- Temperature
- Oxygen management
5. Winery Planning and Design, Edition 16, Available.
This publication, which I edited, is in CD format and is the result of a number of workshops and short courses I have organized on various aspects of winery planning in various regions of the country. The information provided is from a number of authoritative sources and is not linked to specific geographic regions.
Winery Planning and Design, Edition 16 is available through the industry trade journal Practical Winery and Vineyard (at http://www.practicalwinery.com/bookshelf.htm, phone 415- 479 5819, email: ).
Subject headings include:
- Winery Business Planning
- Winery Economics
- Winery Public Relations
- Winery Design Considerations
- Gravity Flow Design
- Wine Caves
- Examples of Winery Designs
- Winery Equipment
- Winery Architects and Tasting Rooms
- Sustainable Designs and Design Considerations
- Water and Waste Water
- Winery Sanitation, Winery Lab, HACCP Planning
- TTB
- Wine Distribution
- Winery Software and Consultants
A full listing of the CD index is available on the Enology–Grape Chemistry Group at www.vtwines.info. Click “Winery Planning and Design.”
References
Boulton, R. (2001) The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 52, 67-87.
Kennedy, Jim, David Jeffery, Leigh Francis, and Markus Herderich. 2011. Science Looks at Sensory Perception Effect. Aust. and New Zealand Grape Grower and Winemaker. Oct. p 61-69.
Jackson, R. Wine tasting, a professional handbook. 2002. Academic Press. 291pp.
McCord, Jeff. 1998. StaVin Inc. Research presentation.
McRae, J.M.; Falconer, R.J.; Kennedy, J.A. (2010) Thermodynamics of grape and wine tannin interaction with polyproline: Implications for red wine astringency. J. Agric. Food Chem. In review.
Sáenz-Navajas, M.-R; Campo, E.; Fernández-Zurbano, P.; Valentin, D.; Ferreira, V. (2010) An assessment of the effects of wine volatiles on the perception of taste and astringency in wine. Food Chem. 121, 1139-1149.
Zoecklein, B. W., Ken Fuglesand, B.H. Gump and F.S Nury 1999, 2005. Wine Analysis and Production. Kluwer Academic/Plenum Publishers.
All past years’ Enology Notes are still available. Use this link to jump to the most recent year's index: 2010 Enology Notes Listing.![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Enology-Grape Chemistry Group's web site at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes listserve send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce
Zoecklein
Professor and Enology Specialist Head Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Cell phone: 540-998-9025
Email:


