Enology Notes #149
All past years' Enology Notes are still available. Use this link to jump to the most recent year's index: 2009 Enology Notes Listing.
Enology Notes #149, July 16th, 2009 - Link to PDF
To: Grape and Wine Producers
From: Bruce Zoecklein, Head, Enology-Grape Chemistry Group, Virginia Tech
Subject:
- France Technical Study Tour
- Winery Tasting Room Design and On-Site Marketing Meeting
- Yeast Assimilable Nitrogen (YAN) Measurements
- Controlling Wine Oxidation
- Virginia Tech’s Enology Service Laboratory – Metal Analysis
- 34th Annual ASEV-ES Conference and Symposium
1. France Technical Study Tour. The 2009 Wine Industry Technical Study Tour is an 8-day tour of Southern France to meet and interact with key grape growers, winemakers and technical support personnel. The dates are December 1– December 9, 2009.
The objectives:
This is a unique opportunity to visit and participate in technical discussions on a wide variety of practical grape and wine production topics. This Study Tour is designed for personnel in the commercial grape and wine industry, and includes:
• Visit the flagship wineries and vineyards in Southern Rhône, Provence, and Languedoc.
• Visit the three leading institutions for practical grape growing and winemaking: Institut Coopératif du Vin (the largest wine consulting company in the world), InterRhône (the Rhône research and experiment center), and Centre du Rosé (the experiment center for Provence wines).
• Visit the largest equipment show for vineyards and wineries in the world: SITEVI (Salon International des Techniques et Equipements Viti- Vinicoles).
• A unique opportunity to meet and exchange ideas with top wine growers of Rhône varieties, in some of the best and most unique places (Muscatà Petits Grains at Lunel, Grenache at Gigondas, Mourvèdre at Bandol, Clairette at Palette).
• A unique culinary opportunity for fine food and fine wines.
This 2009 Technical Study Tour will be led by industry technical experts: Pascal Durand, Professor, University of Burgundy and Director of the International Marketing postgraduate program of SupAgro Dijon, Dijon, France. Bruce Zoecklein, Professor of Enology, and Head of the Enology–Grape Chemistry Group at Virginia Tech, Blacksburg, VA. Keith Patterson, Professor of Viticulture, CalPoly, San Luis Obispo, CA.
This study tour is restricted to wine industry professionals only. For additional details, including the full itinerary, go to www.vtwines.info.
2. Winery Tasting Room Design and On-Site Marketing Meeting. A meeting on Winery Tasting Room Design and On-Site Marketing is scheduled at Surry Community College, Dobson, North Carolina, on Saturday, November 14, 2009.
Organized by Dr. Bruce Zoecklein, Head, Enology-Grape Chemistry Group, Virginia Tech, and hosted by Surry Community College, this one-day program will bring some of the country’s leading marketers and winery tasting room designers together to discuss and highlight the relationships between design components and wine marketing.
The program will include the following topics:
- Winery Design Components and On-Site Marketing
- Winery Tasting Room Design
- Strategies for On-Site Marketing
- Common Winery Marketing Mistakes
- Winery Sustainability and Branding
- 'Designing’ Your Tasting Room Staff
- Optimizing Winery Tourism
- Winery Business Planning and Cash Flow
Speakers committed to date include:
Craig Root, Craig Root & Associates, Napa, CA. Craig has designed a number of Napa Valley winery tasting rooms.
Paul Wagner, Balzac Communications and Marketing, Napa, CA. Paul is an author and leading wine marketing consultant.
Barbara Lindblom, Winemaker Consultant and Sensory Specialist, Santa Rosa, CA.
Patty Held, Patty Held Consulting Services, Herman, MO. Patty has 25 years’ experience in wine marketing, including tasting room staff training.
Jerry White, Agricultural Economist and Professor Emeritus, Cornell University. Jerry has an extensive background in winery economics and has aided many producers on issues of evaluating cash flow and marketing.
Registration materials will be posted at www.vtwines.info and www.surry.edu.
3. Yeast Assimilable Nitrogen (YAN) Measurements. The Enology Service Laboratory is again offering pre-harvest and harvest yeast assimilable nitrogen analysis for the 2009 season. We measure YAN, NH3 and Arginine. The sample submission procedure is as follows:
- Email the VT Service Laboratory at to request berry processing bags and/or sampling bottles.
- Include name, company, mailing address, and the number of berry processing bags and/or sampling bottles required. There is a minimum order of 5 sampling bottles.
- Collect representative juice samples at harvest, or grape berry samples pre-harvest. Representative vineyard samples can be collected up to 5 days pre-harvest. Do not send unprocessed berries to the lab. Berry samples should be crushed in the processing bags and the juice poured into the sampling bottles.
- Fill sample bottles to the indicated level, and mix thoroughly to dissolve the green-colored preservative which comes in the bottle.
- Ship samples for next day/early morning delivery to the Enology Service Laboratory (shipping delays impact analysis results).
- Results will be posted on your secure website the same day samples are received.
There is a $1 charge for each sample bottle. Fermentable Nitrogen analysis (YAN, NH3, and Arginine) is $25 per sample. The juice analysis panel (Brix, YAN, Malic Acid, pH, and TA) is $60 per sample.
For information on how to collect representative samples, see On-Line Publications, Maturity Evaluation for Growers, at www.vtwines.info. For information regarding sample processing, see below.
Measurement of YAN is a barometer to the nitrogen status of the plant. See Enology Notes at www.vtwines.info.
a. YAN, a Review. The nitrogen required by yeast to conduct a healthy fermentation includes two forms: ammonia N, and a group of amino acids referred to as alpha-amino acids, or free amino nitrogen (FAN). Together, the sources contribute the nitrogen utilized by yeast, referred to as yeast-assimilable nitrogen (YAN), or sometimes referred to as fermentable nitrogen.
The minimum yeast assimilable nitrogen required is approximately 140 mg/L for a 21-degrees Brix juice, and perhaps 250 mg/L for a 23-degrees Brix juice. However, it should be noted that these concentrations are broad-based generalizations for several reasons:
- The nitrogen level required to optimize fermentation is highly yeast-strain specific, governed largely by the genetics of the yeast.
- It may be that the qualitative makeup of FAN amino acids, not simply the total yeast assimilable N, is the most important factor. The significance of the qualitative nature of YAN may help to explain so-called reductive grapes, varieties that have a greater tendency to produce sulfur-like off odors. It also helps to explain seasonal and block differences in sulfur-like off odor production.
- A low concentration of yeast assimilable nitrogen is often coupled with deficiencies in important micronutrients required for optimum yeast performance.
b. Grape Sample Processing Method and YAN. There is a relationship between juice extraction methods and YAN nitrogen. Amino acids are not equally distributed in the grape berry. For example, with mature Cabernet Sauvignon, about 8% of the total is in the seeds, 15% in the skins, and 77% in the pulp. It would seem that the method and degree of separation of the pulp juice from the skins would not have a large quantitative effect on YAN concentration, but it does. There is a significant qualitative influence. As such, the following contrasting production schemes usually result in very different YAN concentrations in the juice.
- Whole cluster pressing vs. crush and drain of whites
- Bleeding vs. non-dejuicing reds
- Short- vs. long-vatted reds
The two grape amino acids present in the greatest concentration are proline and arginine. Proline cannot be used by the yeast, while arginine can. Indeed, because it has four atoms of N per molecule, arginine is a very good source of fermentable N.
In the case of Cabernet Sauvignon (and likely most other red varieties) the ratio of arginine to proline is much greater in the skins than the pulp. In other words, the pulp juice which is taken off during bleeding has a relatively high concentration of proline (approximately 55%) which cannot be used by the yeast, and a small concentration of the more potent amino acid, arginine, and others needed to carry out a healthy fermentation. The lower incidence of incomplete fermentation in red, compared with white, musts supports the concept that the slow release of nitrogen from grape skins during fermentation is important.
In order to determine the maximum YAN concentration in fruit or must, the sample should be completely macerated, yet seed breakage avoided. Evaluating lightly pressed free-run juice usually provides a low YAN reading, not indicative of the YAN potential of the fruit or must.
4. Controlling Wine Oxidation. Why do some wines seem to fade much more quickly than expected? There can be a number of reasons for this; many have been outlined in previous editions of Enology Notes. Dr. Andy Waterhouse has aided our understanding of wine oxidation and the role of metals. The following is a brief review.
When wine compounds combine with oxygen, they can pick up one or more oxygen atoms and become “oxidized.” These new compounds are now different, and can have different sensory characteristics. For example, ethanol can be oxidized to acetaldehyde and further, to acetic acid, each with very different sensory features.
Similarly, polyphenols can be oxidized to quinones, and metals such as copper, iron, and manganese can be transformed from Cu+ to Cu2+, Fe2+ to Fe3+, and Mn2+ to Mn3+, respectively, with potential sensory changes. These same multivalent metals can also act as catalysts in oxidation reactions. Oxygen can complex with these metals, and they have been identified in intermediate oxidation products.
The traditional view of wine oxidation is coupled oxidation, where phenols are oxidized to form quinones (brown phenols) and produce hydrogen peroxide. The hydrogen peroxide goes on to react with a dominant species in wine, such as ethanol, to form acetaldehyde as depicted below.
Coupled Oxidation
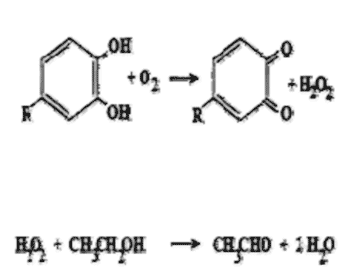
Because oxidative degradation results in the loss of aroma/flavor, the concept of hyper-reduction has developed. As reported in Enology Notes #102, hyper-reduction involves processing steps to help minimize oxidative degradation by keeping wines in a reduced or low oxygen state. How well anaerobic processing actually works in helping to control oxidative degradation depends on the methods, the variety, and the metal content of the wine.
Iron and copper are both strong oxidizers. The potential oxidizing effect is illustrated by the Fenton-type reaction:
H2O2 + Fe+2 → Fe+3 + OH- + OH*
The OH*, or free hydroxyl radical is formed in the presence of iron and likely other metals. It is the most oxidative species that can react with a number of wine components, impacting the sensory features and longevity. An important implication of the presence of hydroxyl radical is the formation of numerous aldehydes and ketones from oxidation of alcohols. These alcohol derivatives react with flavonoid phenols, creating linkages that may help stabilize color and create bonds between tannins, and between tannins, proteins, and polysaccharides. The control and extent of oxidative reactions is the important issue.
It is likely that a relatively high concentration of metals inhibited practices such as microoxygenation in the past. When I first entered the industry it was not an un- common practice to add citric acid to all wines to help bind or chelate iron to help prevent iron casse formation. High concentrations of iron, for example, can render a wine “over the hill” following only limited oxygen exposure.
The formation of the reactive hydroxyl radical (OH*) requires a metal catalyst such as Fe+2. The Fe+3 produced in the above reaction is converted back to Fe+2 to continue the cycle. This conversion is caused by certain phenols. As such, phenols are acting to accelerate the oxidation process. Additionally, antioxidants do not have any controlling impact on the rate of this reaction.
It is important that winemakers understand the sources of metal pick-up (equipment, fining agents, additives, water) and the possible role that metals, such as iron, can have on their wines.
5. Virginia Tech’s Enology Service Laboratory – Metal Analysis. As indicated in Enology Notes #148, Virginia Tech’s Enology Service Laboratory can determine metal concentrations. As stated above, this is becoming increasingly important as we learn more about the reaction of metals, such as iron and copper, and their role in promotion of wine oxidation.
6. 34th Annual ASEV-ES Conference and Symposium. Wines and Vines in a Changing Climate: Future vineyards in the 21st century will be planted in an environment that will demand increasing quality, that involves global warming and climate change, and will be subject to potential labor shortages. Presentations will address how these factors will affect future vineyard establishment and management in addition to quality winemaking practices.
Quail Hollow Resort
Painesville, OH
July 20-22, 2009
http://www.nysaes.cornell.edu/fst/asev/
![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Enology-Grape Chemistry Group's web site at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes listserve send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce
Zoecklein
Professor and Enology Specialist Head Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Cell phone: 540-998-9025
Email:


