Enology Notes 2004
Back Issues:
Click here for
Enology Notes from 2000 (numbers 1-11)
Click here for
Enology Notes from 2001 (numbers 12-35)
Click here for
Enology Notes from 2002 (numbers 36-67)
Click here for Enology Notes
from 2003 (numbers 67-84)
Enology Notes from 2004 (number 97 onwards) {Note: the links below take you to a different page}:
- Structural Balance and Mouthfeel, continued: Yeast Fining; American Society for Enology and Viticulture Annual Meeting and International Symposium; Research Activity.
- Winery Planning and Design Workshop, Gum Arabic.
- Winery Planning and Design Workshop, and American Society for Enology and Viticulture, Eastern Section, Technical Meeting and Symposium.
- Research and Extension at a Crossroads and Winery Planning and Design Workshop
- Norton Roundtable, American Society for Enology and Viticulture - eastern section meeting, Laboratory Services, and Research and Extension at a Crossroad (continued)
- Red Wine Production Considerations and Norton Roundtable; American Society for Enology and Viticulture Annual Meeting and Symposium
- Lysozyme; ASEV-ES Annual Meeting and Symposium; Norton Roundtable Meeting
- Brettanomyces; Laboratory Services; New Books
- Juice and Wine Analysis Short Course, Wine Barrel Survey, New Edition of Winery Planning and Design Manual Available, Issues for the Season, Volatile Sulfur Compounds
- Herbaceous Character in Red Wines; Environmental Taints; Upcoming Extension Program; New Edition of Winery Planning and Design Manual Available
- Oxidation Reduction Potential; Upcoming Enology Extension Programs
- Oxidation Reduction continued; Wine Closures; Upcoming Extension Programs; New Zealand Travel
Enology Notes #96 December 20, 2004
To: Regional Wine Producers
From: Bruce Zoecklein, Head, Wine/Enology-Grape Chemistry Group, Virginia Tech
Subjects: Oxidation Reduction continued; Wine Closures; Upcoming Extension Programs; New Zealand Travel
It is the molecular form of a compound (such as acetic acid, for example) that is volatile, and therefore responsible for aroma. The ionized forms (such as acetate) are not volatile and do not contribute to aroma. Thus, a shift in a wine’s oxidation-reduction (redox) potential may produce more or less of the volatile form of a number of compounds, creating a significant sensory impact.
Therefore, redox potential influences sensory attributes, including wine aging. After bottling, there is a progressive decrease in redox potential. The intensity of the bouquet is linked to the level of redox potential, which depends on the wine, temperature and efficacy of the closure.
This edition continues a series on oxidation-reduction potential. This will serve as an introduction to important interrelated issues which will be reviewed in the future: aroma, volatile sulfur compounds, and bottle age development.
A portion of the following was authored by my colleague and coauthor of Wine Analysis and Production, Dr. Barry H. Gump, Viticulture & Enology Research Center, California State University, Fresno.
Oxidation and Reduction. When oxygen combines with wine compounds, they can pick up one or more oxygen atoms and become "oxidized". These new compounds are now different, and can have different sensory characteristics. For example, ethanol can be oxidized to acetaldehyde and further to acetic acid, each with very difference sensory features.
Similarly, polyphenols can be oxidized to quinones, and metals such as copper, iron, and manganese can be transformed from Cu+ to Cu2+, Fe2+ to Fe3+, and Mn2+ to Mn3+, respectively, with potential sensory changes. These same multivalent metals can also act as catalysts in oxidation reactions. Oxygen can complex with these metals, and they have been identified in intermediate oxidation products.
Reduction is the opposite of oxidation; it is a process whereby compounds may lose oxygen atoms. Since wine is fermented by yeast through an anaerobic process (without oxygen), a number of reduced compounds are produced. Reduced sulfur and nitrogen compounds, in the form of hydrogen sulfide and mercaptans (ammonia and amines), are known particularly for the negative characters they impart to wines. Thus, it is possible to have a wine with an unpleasant and undesirable "reduced" character.
The redox processes
In the processes of oxidation and reduction, referred to as redox reactions, various wine components in oxidized or reduced forms react and change from one form to the other. These reactions are always coupled: for oxidation to occur, reduction must also occur.
Along with a gain or loss of oxygen, a transfer of electrons occurs from one compound to another. Atmospheric oxygen can take a pair of electrons from another compound and cause this compound to be oxidized, and the oxygen atom with its newly acquired electrons is reduced (picks up two extra electrons for each oxygen atom) and becomes O2- such as found in water, H2O, or ethanol, CH3CH2OH.
Thus, a component is oxidized when it loses electrons (or gains oxygen atoms and/or loses hydrogen atoms); it is reduced when it gains electrons (or loses oxygen atoms and/or gains hydrogen atoms). A compound that loses electrons must give them to another compound or atom. This process of transferring electrons from one species to another is the actual basis of a redox reaction.
Wine oxidation is not a single process; it involves a sequence of oxidation/reduction reactions between various wine constituents. Wine is a complex chemical system composed of many potential redox pairs (pairs of compounds that interact through the oxidation-reduction process).
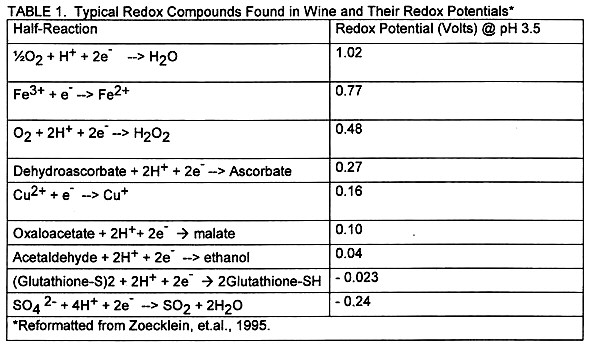
Redox reactions, with their transfer of electrons between various chemical components, occur throughout fermentation and continue until consumption of the final product (actually, that starts another series of redox reactions we call metabolism). These reactions occur when one compound with a relatively greater affinity for electrons attracts one or more electrons from another compound, which has a lower affinity for electrons.
An oxidizing agent, such as molecular oxygen, causes other compounds to become oxidized. The oxygen in turn is reduced by collecting electrons from the other compound. Common reducing agents in wines, such as sulfur dioxide, ascorbic acid, and wine phenols, give up electrons to other compounds (oxygen, for example), causing them to be reduced. The reducing agent becomes oxidized as a result of this process; for example, sulfur dioxide is oxidized to sulfate.
When a wine is exposed to oxygen, oxidation can occur. Depending on the extent and rate of oxygen contact, changes in the sensory characteristics will be more or less noticeable. We encourage the particular redox reaction between oxygen and sulfur dioxide in our wine processing because, by binding free oxygen, the sulfur dioxide prevents oxidation of other wine components.
Sulfur dioxide also binds up free carbonyl (i.e., acetaldehyde) compounds produced during oxidation, reducing their concentrations below sensory threshold. This minimizes the effects of incipient oxidation and the attendant deterioration of wine aroma.
Wine Closures. In November, I attended the First International Screwcap Symposium, in Marlborough, New Zealand, where Peter Goddon of the AWRI offered this interesting quote, "Closure variation may have a greater impact on wines and wine quality than the widely discussed and debated terroir issues. After about a month in the bottle, wines under different types of closures will never be the same." The work of the Wine/Enology-Grape Chemistry Group supports this observation. Closures change wine dramatically, not just at different rates, but they cause wines to age differently.
The degree of variation among wines bottled with cork closures has increased the interest in alternatives. Screwcap closures are now offered by one of the Virginia bottling line services. This suggests the need to review some of the technical issues regarding wine closures.
There were several other interesting comments and quotes offered at the Screwcap Symposium:
"I believe wines bottled with corks will be in the minority by 2015…More and more state-of-the-art wineries are moving to screwcaps for wines that need to be consumed within 3 to 4 years of the vintage (about 95 percent of the world's wines). Look for this trend to accelerated." -Robert Parker. "Parker Predicts the Future." Food & Wine, October 2004.
Not everyone shares this view:
"What can't be remedied is the tinny metal closure on the wine. I'm afraid the screwcap will have to go…In short, I believe screw tops will be limited almost exclusively to inexpensive wines and for a long time." -Ben Giliberti. Washington Post, October 13, 2004.
"Over 80% of the households in the USA do not have a corkscrew." -Michael Franz, oral presentation, First International Screwcap Closure Symposium.
In the very traditional wine industry of France only three well-know producers bottle their ultra-premium wines in screwcaps: Andre Lurton, Paul Blanck and Michael Laroche. This may be rapidly changing.
Michael Laroche tells the story of his motivation for bottling his very expensive Chablis in screwcaps.
"In April 2004, I tasted 40 theoretically identical [cork closure] bottles of 2001 Chablis (same wine, same vintage, same bottling date). Of these 40 bottles, 15 were judged totally unacceptable. 22 bottles were commercially acceptable, although with significant variation. Only 3 bottles of 40 were considered optimum quality!"
The success of screwcaps for the New Zealand wine industry over the last four years has been nothing short of revolutionary. To what extent should the Virginia industry adapt to screwcaps, and for what wines? The Wine/Enology-Grape Chemistry Group has evaluated the impact of various closures on wine aroma volatiles. Due to the interest in wine closures, some important issues will be reviewed in this and subsequent editions.
I will conduct a closure review workshop this winter or early spring. At that program, I intend to present wines aged with different closure types, including natural cork, various synthetics and screwcaps. The date, time and location will communicated.
It is essential to understand that changing closures is not simply a cosmetic issue. The New Zealand Screwcap Initiative was established in 2001 to instigate and evaluate closure research, including winemaking practices which may be impacted by closure selection. This includes the role of closures on wine aging and sulfide production. Both topics will be reviewed in subsequent editions of Enology Notes. More to follow.
Wine Filtration Workshop. The Wine/Enology-Grape Chemistry Group, in conjunction with Pall Corporation, will offer a one-day juice and wine filtration workshop, February 10, 2005, at Horton Cellars, from 10:00 am to 4:00 pm. Fee is $35. See Enology Notes #95 for details.
Wine Fining. The Wine/Enology-Grape Chemistry Group, in conjunction with Scott Laboratory, will offer an afternoon advanced workshop on juice and wine fining, February 28, 2005, at White Hall Vineyards, from 12:30 to 4:30 pm. Fee is $30. See Enology Notes #95 for details.
Enrollment for both programs is Limited and Restricted: Registration is secured only after payment has been received. For questions, contact Terry Rakestraw at 540-231-6805 or .
New Zealand Travel. I would like to thank the New Zealand Society for Viticulture and Oenology, the VinoTech Group, and Pacific Rim Enology Services for the invitation to speak in New Zealand this past November. Special thanks to Lisa Van de Water of Pacific Rim Enology Services for being such a gracious host, and arranging and providing travel, cultural, and recreational activities (fly fishing!).
Happy Holidays!
![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Wine Enology Grape Chemistry Lab's website at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes list, send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce Zoecklein
Professor and Enology Specialist Head Wine/Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Email:
