Enology Notes
Enology Notes #120, November 2, 2006
To: Regional Wine Producers
From: Bruce Zoecklein, Head, Enology-Grape Chemistry Group, Virginia Tech
Subject: Tannin Polymerization and Color; Enology Service Lab New Equipment - Segmented Flow Apparatus, Equipment Donation; Juice and Wine Analysis Short Course; Winery Planning and Design Workshop; Winery Planning and Design CD Available
1. Tannin Polymerization and Depolymerization. Historically, winemakers have thought the distribution of various sized tannins depended mainly on the degree of fruit maturity.
It was assumed that as fruit maturity increased, there was an increase in the degree of polymerization (DP number) or extent of tannin binding. We further assumed that a certain level of fruit maturity was needed to create a certain size tannin molecule, and that tannins simply got bigger and more polymerized as maturation progressed.
We now know that condensed grape tannins are not stable, and that in the acid environment of wine, they can undergo polymerization as well as hydrolysis or depolymerization.
Therefore, during wine processing there can be modifications in the DP number. This helps to explain some of the sensory changes that occur during wine aging.
2. Color and Tannin Polymers. Much of the fruit harvested this season in the Virginia region appears to be relatively low in red color. The question is, what is the relationship between the anthocyanin content of the fruit and resultant wine color?
As outlined in previous editions of Enology Notes (#85, 118), spectral color in wine is a function of three elements:
- anthocyanin concentration
- concentration of cofactors, or certain non-colored compounds, which bind with anthocyanins to cause copigmentation
- polymeric pigments
In grapes and wines, anthocyanin pigments can be either free molecules, unbound monomers, or associated with other phenols, such as tannins, to form polymeric pigments.
Binding with other phenols can impact the sensory quality of anthocyanins by increasing color stability, and by changing the spectral color from bright red to brick-red. Indeed, the formation of pigment polymers is a reason why young red wine color changes.
Several years ago, an interesting red wine color phenomenon was noted, which is illustrated below (Ribereau-Gayon and Glories, 1983).
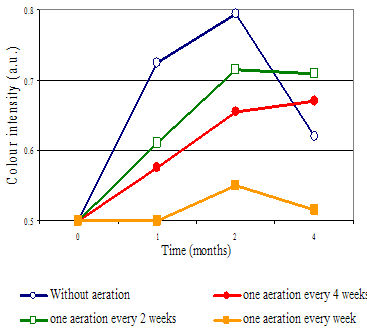
Wines made without any air or oxygen exposure developed more red color initially, but soon the color declined. In other words, the red color was not stable.
Conversely, red wines produced with some air exposure developed more color and color stability, while too much exposure demonstrated loss of color.
Clearly, oxygen was playing a strong role in red wine color formation and stability. The question is, what is the role of oxygen in color, and how much oxygen is needed for optimizing color?
Oxygen helps to form cross-links between anthocyanins and tannins, thus helping to stabilize color. How much oxygen is desirable depends on the percentage of anthocyanins in the monomeric (non-polymerized) form, among other factors.
In solution, the unbound anthocyanin molecule is positively charged, which enables it to absorb light, and thus have color. Anthocyanins have a carbohydrate (sugar), usually glucose, attached (esterified) at the 3-position. The presence of this sugar helps the anthocyanin maintain solubility and stability.
If the sugar is hydrolyzed or lost, the solubility and stability decrease. What is equally important for red color is the association of this molecule with tannins and other phenols. There are several mechanisms (oxidative and non-oxidative) by which pigment polymers are formed.
One example illustrates the role of tannin depolymerization. If a tannin of DP-10 size is hydrolyzed under acidic conditions in wine, it can break up to shorter lengths, such as two DP-5s.
In such a case, the hydrolysis would produce one electron-neutral and one positively-charged tannin.
Depending on the concentrations of available tannins and anthocyanins, the positively-charged tannin will react with either another tannin or an anthocyanin molecule. This explains why the ratio of tannin to anthocyanin in the must is important, regarding color and color stability (see Enology Notes #116, 118).
If it reacts with another tannin, a longer non-colored polymer will be formed. However, the process is different if it reacts with an anthocyanin. If the anthocyanin reacts with a tannin, this new larger anthocyanin-tannin polymer acts as an electron sink and stabilizes color.
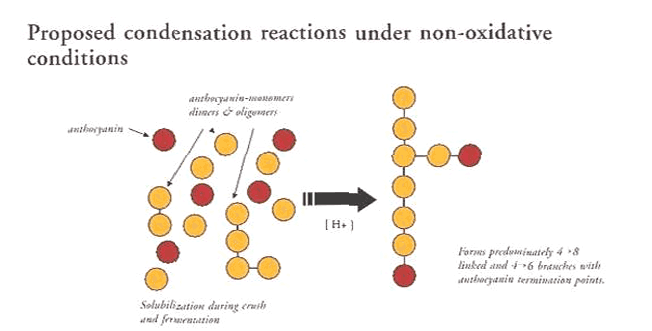
Source: McCord, 1990
A unique aspect of this binding is that the anthocyanin molecule is no longer available to react, and the anthocyanin acts as a terminus for any further reaction at that end of the polymer (McCord, 1990).
In addition to the direct reaction described above, anthocyanins and tannins can be linked by aldehydes. This is the basis for microoxygenation’s impact on color and stability.
Aldehydes are able to act as cross-linkers, binding anthocyanins and tannins. Many aldehydes can be derived from toasted oak. Aldehydes are also produced as a result of ethyl alcohol oxidation, which occurs during wine microoxygenation.
Thus, microoxygenation (including splash racking) of very young wines (when there is a high percentage of monomeric or unbound anthocyanins) can aid in color and color stability, as a result of oxidative formation of anthocyanin-tannin pigment polymers.
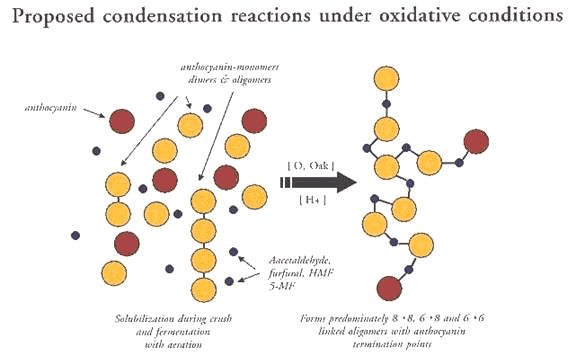
Source: McCord, 1990
As these two figures indicate, there is a structural difference in tannins cross-linked with aldehydes, versus reductively-formed tannin pigment polymers.
Anthocyanin-tannin polymers stabilize themselves through stacking and aggregation. Pigment polymers formed differently (oxidatively vs. non-oxidatively) may aggregate differently (see figures).
The many kinks added into the polymer by cross-links with aldehydes may prevent tight association.
More to follow.
3. Enology Service Lab New Equipment - Segmented Flow Apparatus. A Segmented Flow Apparatus is currently being installed in the Virginia Tech Enology Analytical Services Laboratory. This type of equipment is relatively new to enological analysis. A wine sample is drawn into the machine and divided into segments or bubbles. These bubbles are individually analyzed for wine components. Initially, we will use this system for sulfites and volatile acidity.
Sulfites – The Segmented Flow Apparatus can measure both free and total sulfites. The advantage is in both the method and speed. Many wineries use Ripper titration. The drawback for this procedure is that certain wine components interfere with the results, providing false high readings.
The TTB-accepted method for the accurate reporting of both free and total sulfites is Aeration/ Oxidation. The Segmented Flow Apparatus is unique in that it uses a combination of dialysis membranes and heat cartridges to perform the Aeration/Oxidation procedure on a small, although highly accurate, scale. Performing the standard procedure on a wine sample in duplicate takes approximately one hour. The Segmented Flow Apparatus reduces this to approximately one minute.
Volatile Acidity – Volatile acidity analysis is the quantification of the total volatile acids present in wine, but it is reported as grams of acetic acid per liter. This can be slightly deceiving, in that there are more volatile acids in wine than just acetic acid.
The common method for measuring these acids is to steam distill the wine and collect the volatile acids that are released, using a Cash Still. To perform this procedure takes about 45 minutes. The Segmented Flow Apparatus performs the same method, on a smaller scale, in less than one minute.
Another advantage of Segmented Flow is its expandability to monitor a wide range of wine components. This range is expanding monthly as the technology improves and is verified.
4. Equipment Donation. The Enology-Grape Chemistry Group would like to thank Bernd Jung of Chester Gap Cellars, Front Royal, VA, for the generous donation of a variable-capacity fermentation/storage tank for our research winery.
5. Juice and Wine Analysis Short Course. The Enology-Grape Chemistry Group will offer its annual two-day juice and wine analysis short course on January 10 and 11, 2007. This program will be a hands-on, practically-oriented laboratory course. It will be conducted in the teaching laboratory of the Food Science and Technology Building at Virginia Tech.
This program will include the following:
- Establishing a winery laboratory
- Good laboratory practices/HACCP planning/precision and accuracy
- Fruit processing basics
- Maturity indices
- pH basics
- Titration and titratable acidity
- Fermentable nitrogen
- Sugars
- Pectins/glucans/filterability
- Alcohol
- Protein stability
- Bitartrate stability
- Oxidative stability and aldehydes
- Organic acids
- Volatile acidity
- Sulfur dioxide and sulfur-containing compounds
- Dissolved oxygen
- Carbon dioxide
- Introduction to remission photometry
- Virginia Tech’s analytical lab service
Registrants will participate in hands-on analysis. Analysis will be supplemented with a laboratory manual and discussions concerning the practical winemaking significance of each test.
Enrollment is limited: Registration preference will be given to Virginia bonded winery owners and representatives, and prospective Virginia winery owners, who register BEFORE WEDNESDAY, NOVEMBER 8, 2006.
Preference will be given to those already in the commercial wine industry.
Cost: $450 per person. Make checks payable to: Virginia Tech Foundation. On the memo line of your check, please note Juice/Wine SC AND the name of the winery you represent. Mail to:
Terry Rakestraw
Department
of Food Science & Technology
25-A FST Bldg.,
Virginia Tech - 0418
Blacksburg,
VA 24061.
6. Winery Planning and Design Workshop. On March 5, 2007, we will be conducting a Winery Planning and Design Workshop, in conjunction with Wineries Unlimited. This year’s program will be at the Valley Forge Convention Center, just outside of Philadelphia.
This one-day meeting will include a variety of topics with an emphasis on design considerations:
Winery Design
- Winery Design Considerations
- Winery Layout
Winery Equipment
- Equipment Considerations
- Equipping a Small Winery
- Fermentation and Storage Vessel Considerations
- Wine Caves
- Gravity Flow Winery Designs
Examples of Winery Designs
- Winery Architecture
- Winery Designs, Case Studies, and Winery Tasting Room Design
Wastewater Treatment
Also included: winery business planning, winery economics, and refrigeration.
For registration information see http://www.wineriesunlimited.com.
7. Winery Planning and Design CD Available. The Enology-Grape Chemistry Group’s comprehensive 200-page review covers the essential planning and design considerations of critical importance in winery establishment. The CD covers the topics listed below. Each subject area is reviewed in a simple-to-read outline form.
The Winery Planning and Design CD is available through Practical Winery and Vineyard magazine for $95. Email them at , or call (415) 479-5819.
Winery Planning and Design CD subject areas:
Winery Business Planning
- Elements of a Successful Business Plan
- Tips for a Successful Executive Summary
- Small Business Development Center Business Plan Outline
- Business Plan Worksheet
Winery Economics
- The Economics of Various Size Wineries, and Economies of Scale
- Cash Flow Review
- Starting a New Winery
Winery Design
- Winery Design Considerations
- Winery Layout
Winery Equipment
- Equipment Considerations
- Equipping a Small Winery
- Fermentation and Storage Vessel Considerations
- Wine Caves
- Gravity Flow Winery Designs
Examples of Winery Designs
- Winery Architecture
- Winery Designs and Case Studies
- Winery Tasting Room Design
Green Wineries and Sustainable Winegrowing
Winery Refrigeration
Winery Water Requirements
Winery Wastewater Treatment
The Winery Laboratory and HACCP Planning
Winery Legal Issues
![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Enology-Grape Chemistry Group's web site at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes listserve send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce
Zoecklein
Professor and Enology Specialist Head Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Cell phone: 540-998-9025
Email:
