Enology Notes
Enology Notes #113, May 1, 2006
To: Regional Wine Producers
From: Bruce Zoecklein, Head, Enology-Grape Chemistry Group, Virginia Tech
Subject: Volatile Sulfur Compounds: Impact on Aroma, Flavor, and Texture, and Practical Management
I. Volatile sulfur compounds (VSCs) make significant contributions, both positive and negative, to wine aroma, flavor, and mouthfeel, due to their reactivity and low sensory thresholds. The production of unpleasant VSCs, referred to as reductive or sulfur odor defect, is considered to be one of the most significant winemaking problems worldwide.
It is essential that premium winemakers understand the nature of VSCs, and effective management procedures for dealing with reductive odor defect. This is another in a series of reviews on this important subject (see Enology Notes #70, 71, 76, 79). A slide show on this subject, originally presented in New Zealand, is available; from the homepage, click on “On-line Publications & Current Topics,” then on “Volatile Sulfur Compounds.” In the current issue, the following will be highlighted:
- Important VSCs
- Sensory contributions of VSCs
- Chemical nature of VSCs
- Mechanisms of VSC production
- Factors impacting VSC production
- Remedial action steps
Although there are no authoritative records, there are those who believe the problem of reductive odor defect is increasing. Is that true, and if so, why?
Many sulfur-containing compounds contribute positively to wine complexity and varietal character. This is notably true for varietals such as Sauvignon blanc, Chenin blanc, Petite Manseng, Riesling, and Gewürtztraminer (see Enology Notes #102, 110).
Volatile sulfur compounds that contribute to reductive odor defect, such as hydrogen sulfide and mercaptans, can have the opposite impact by lowering varietal intensity. This loss of intensity is greater in the presence of methoxypyrazines (herbaceous odors), which provide an odor synergism.
Most winemakers view sulfur odor defect only in terms of olfactory sensations. However, off-odor sulfur-containing compounds can have a large impact on wine mouthfeel. As discussed in previous editions of Enology Notes (#74, 85, 86), mouthfeel balance can be viewed as the following palate balance relationship. Increases or decreases on one side of this ‘equation’ impact the sensory intensity on the other side, thereby impacting the perception of balance.

Yeast metabolism and reductive odor defect. Yeasts can utilize elemental sulfur, sulfate, sulfide, sulfite, thiosulfate, and organic sources of sulfur in grape juice. As a product of sulfate reduction, H2S is an intermediate in the biosynthesis of all sulfur- containing compounds. Consequently, the formation of H2S and other sulfur-containing off-odor compounds in wine is related to both sulfur and nitrogen metabolism.
H2S Formation by Yeast During Fermentation
Sulfur (S0)
Sulfate (SO4-2)
Sulfite (HSO-3) → H2S
Sulfide (S-2)
Organic - S
In a series of regulated steps, sulfate is brought into the yeast cell, and reduced to sulfide via two ATP-activation steps. At this point, sulfide is combined enzymatically with nitrogen-containing carbon precursors to ultimately form cysteine and methionine, two important sulfur-containing amino acids that serve as building blocks for proteins. This sulfate reduction sequence is activated to produce sulfide whenever there is a metabolic demand for cysteine and methionine.
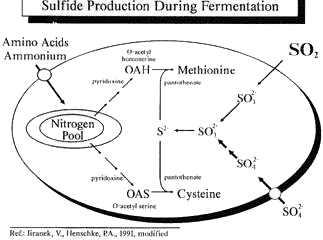
In the absence of intracellular nitrogen, sulfate or sulfite reduction can continue. However, this results in the production of H2S that is not incorporated into amino acids, but liberated from the cell into the medium. Therefore, a high rate of sustained H2S production can be observed in response to yeast cell nitrogen deficiency.
Yeasts not only bring sulfate into the cell, but also sulfite. Unlike sulfate, sulfite (such as sulfur dioxide added pre-fermentation) freely diffuses into the cell. As such, it essentially bypasses the regulatory mechanisms normally controlling the sulfate reduction scheme. This helps to explain why H2S production is greater if too much sulfite is present during fermentation. Addition levels generally should be less than 8 g/HL (see below).
Sensory Features of Reductive Odor Defect. There are nearly 100 sulfur-containing compounds reported in wine. The following is a list of some of the key players responsible for reductive off-odors.
VSC |
Sensory Description |
Sensory Threshold (μg/L) |
Boiling Point (ºC) |
Hydrogen Sulfide |
Rotten egg |
0.5 |
-61 |
Carbonyl sulfide |
Ether |
3.0 |
-50 |
Methyl Mercaptan, Methanethiol |
Stagnant water |
1.5 |
6 |
Ethyl Mercaptan, Ethanethiol |
Onion |
1.1 |
35 |
Dimethyl Sulfide |
Quince, truffle |
10.0 |
35 |
Methionol |
Cooked cabbage |
1200 |
90 |
Diethyl Sulfide |
Ether |
0.9 |
92 |
Dimethyl Disulfide |
Quince, asparagus |
15.0 |
109 |
Diethyl Disulfide |
Garlic, rubber |
4.3 |
151 |
Thioacetic Acid Esters |
|||
H2S contains sulfur in its most reduced, or negatively charged, electron-loaded, form. Mercaptans are common organic compounds. In the presence of air, mercaptans can be oxidized to disulfides, which changes their sensory threshold and character. The oxidized forms are usually less potent.
There are several things to note from the above list. Many of these compounds have different sensory characteristics, including different sensory thresholds. Beyond these differences, this group can be divided into two general categories. Light or low boiling point compounds, those with a boiling point below 90°C, and heavy or high boiling point sulfur compounds, those with a boiling point above 90°C.
This is not an academic distinction. The light compounds have unpleasant odor descriptors, and these can increase post-fermentation. Because of their volatility, their concentration may be lowered by aeration. Some of these, such as H2S and mercaptans, react with copper and can be removed by copper additions, usually in the form of cupric sulfate.
The heavy, or high boiling point, compounds also have a significant role in off-odor defect. They are produced by yeast metabolism during fermentation, not post-fermentation, and they remain stable in the wine post-fermentation. They cannot be removed by aeration, due to their limited volatility, and they do not react with copper. The high boilers represent a large problem for the industry.
A third category of potential sulfur off-odor compounds exits. These are non-volatile precursors, that can change during wine aging to produce volatile sulfur compounds. Compounds like thioacetic acid esters are odorless, but can undergo hydrolysis, or breakdown, to release disagreeable sulfur compounds post-bottling.
It is the quantitative and qualitative composition of these sulfur compounds that provides the impression and intensity of the off odor and off flavors from VSCs. It is also the deciding factor in determining remedial actions. See Zoecklein et al. (1990 or 1999) for an odor screen procedure.
Influencing VSC Odor Defect in Wine. Unfortunately, many of the factors which influence the production of reductive odor defect are not well understood. The following is a partial list.
- Vineyard
- Must nitrogen, sulfate, organic S
- Micronutrients
- O2 management
- Use of enzymes
- Juice turbidity
- Yeast species and strain(s)
- Fermentation temperature
- Fermentor size and shape
- Sulfur dioxide/ascorbic acid management
- Lees management
- MLF
- Light
It has been established that the presence of as little as 1 µg/L of elemental sulfur on the fruit at harvest can produce an H2S concentration above the sensory threshold (0.5 µg/L) in wine. Additionally, it is understood that some synthetic pesticides containing chemically-bound sulfur and/or metals can be sources of reductive odor defect. Beyond spray materials, reductive odor defect appears to be vineyard and block specific. Why?
Some believe the answer relates to the plant’s sulfur metabolism and nitrogen status. Grape nitrogen, required by yeasts to conduct a healthy fermentation, includes ammonia and seven alpha-amino acids, referred to collectively as free amino nitrogen, or FAN nitrogen. Together, these two sources of nitrogen (ammonia and FAN) make up what is needed by yeast, and are referred to as assimilable or fermentable nitrogen.
Fermentable N Requirement. The minimal assimilable or fermentable nitrogen required is about 200 mg/L for a 21 Brix juice, and 250 mg/L for a juice that is 23 Brix. However, there are several considerations that should be noted, regarding listing of minimal fermentable N requirements by yeast. First, a low concentration of N is often coupled with the lack of other important micronutrients. Yeast require inositol, pyridoxine, nicotinic acid, p-aminobenzoic acid, thiamine, pantothenic acid, and biotin, for healthy fermentations. Therefore, a low native concentration of plant N is often not the only issue. Additionally, the true concentration of fermentable N needed is both yeast-specific and impacted by a host of other fermentation variables (see “Factors, Considerations and Recommendations to Produce a Healthy Fermentation,” under “Factors Influencing Fermentation,” through On-line Publications at www.vtwines.info). Finally, it may be the qualitative nature of the FAN amino acids, and not simply the quantitative issue, that is important in the production of sulfur odor defect.
Factors Impacting the Concentration of Fermentable N. There are a host of factors impacting fermentable N, including the following:- Site
- Variety
- Rootstock
- Climate/season
- Soil, soil moisture
- Fertilization
- Cover crops
- Vine density
- Crop load
- Maturity/hang time
- Fruit rot
The nitrogen profile is different in different varietals, and is impacted by rootstock, climate and season, soil moisture, and maturity, among other factors. Seasonal variations and viticultural management practices have a large impact on the N content of the vine.
Nitrogen fertilization affects fruit N, both FAN and ammonia. We know that in some vineyards, the total N concentration in the fruit is only about half the amount reported in the 1970s. At the same time, there appears to be an increase in the incidence of reductive odor defect. Some limit N fertilization as a means of helping to control vine vigor. Changes in soil management systems, such as green cover crops, also deplete N, as does increasing vine planting density.
Fungal degradation is known to lower fruit fermentable N and important vitamins, such as thiamine. Increasing fruit hang time or fruit maturity can deplete N, as can excessive crop load, which can also delay the rate of fruit maturity.
Changes in Fermentable N with Fruit Maturity. Generally, from véraison onward, ammonia decreases, while amino acid concentrations increase, to a point. At harvest, the FAN amino acids’ portion represents 50-90% of the fermentable N. Beyond a certain point, as maturity increases, there is generally a reduction in fermentable N. This has become evident in warm climates, and with increasingly longer fruit hang time. In some cases, this has resulted in increased incidence of reductive odor defect. The other extreme may also be important. Some have observed that less-ripe fruit can increase the concentration of off volatile sulfur compounds.
The two amino acids present in the greatest concentration in the fruit are proline and arginine. Proline cannot be used by the yeast aerobically, while arginine can. Indeed, because it has four atoms of N per molecule (two or three of which may be used by yeast), arginine is a very good source of fermentable N.
Arginine accumulation begins well before véraison and continues during maturity, but plateaus. Proline, on the other hand, increases late in the season (4 weeks post-véraison). High proline is associated with increased maturity and increased vine stress, particularly moisture stress.
Determining Fermentable N. It is essential that fruit or juice be checked for fermentable N prior to fermentation each season. There are several methods of measuring fermentable N. We evaluated the Formol titration, made some changes in the procedure, and compared the results with the other standard methods, NOPA and HPLC of the FAN amino acids, plus ammonia. The procedure is on the Enology-Grape Chemistry Group website at www.vtwines.info. Click On-line Publications, then either Fermentable N, or Prediction of Nutrient Status of Grape Juice.
Processing methodology influences the qualitative nature of must N. Amino acids are not uniformly distributed in the grape. Therefore, both sample processing and winemaking can influence the amino acids extracted. For example, there is a higher ratio of arginine to proline in the skins. The opposite is true for the pulp, where there is more proline and less arginine.
As such, the separation of the pulp juice from the skins, as occurs during whole cluster pressing of whites and bleeding (sangue) of reds, has a large effect on juice N status and the potential for reductive odor defect production.
Fermentable N and Yeast Strains. Determining the optimal concentration of fermentable N depends on several factors, including yeast species and strain. Winemakers select strains for a variety of reasons, most notably for desirable aroma/flavors. Some strains are less efficient users of N, and have higher N requirements. Defining the optimal N concentration for a particular strain is difficult, due to the multi-dimensional nature of fermentations. Yeast strains are also extremely variable in their ability to form VSCs. Complex genetic factors lead to variability in levels and timing of H2S produced by yeast, however, no commercial yeast strain has been isolated or bred that does not produce H2S.
Off-VSC formation in some yeast strains appears to be relatively independent of total fermentable N status over a fairly wide range. However, yeast variability, with regard to reductive odor defect, is greatest under conditions of marginal N or other stress factors. An important influence may not simply be the total fermentable N, but the ratio of certain amino acids in the juice. This appears to be related to the methionine concentration of the juice, and perhaps the ratio of other amino acids. This helps explain why we can have such large variations in the off-VSC formation from vintage to vintage.
Adding Supplemental Sources of N. Off-sulfur compounds are also impacted by the nature of the addition compounds to the fermentor, and their timing. Too high a concentration of added N can cause the following:
- Increased production of off VSCs
- Increase unwanted flora (if added too early or too late)
- Rapid fermentation
- Loss of volatiles
- Decreased complexity
Many winemakers prefer too much N to too little. The theory is that it is better to be approximately right than absolutely wrong. Naturally, there is a problem with simply dumping nitrogen supplements into the fermentor. It can stimulate the growth of unwanted organisms, increase the biomass, and cause too rapid a fermentation. Rapid fermentation can increase aroma loss due to volatility, resulting in the loss of complexity. Furthermore, the addition of ammonia can prevent the appearance of aromatic degradation products from amino acids. Amino acids are an important source of esters that can add to complexity and wine quality.
A supply of nitrogen must be available to the yeast to allow the continuous resynthesis of proteins. If that does not occur, the yeast lose the ability to conduct the fermentation. Therefore, nitrogen additions may be effective in avoiding problem fermentations until about 2/3 of the sugar is utilized. Cells which have passed the point of transcriptional responsiveness will not respond to added nutrients. This means that yeast cell cycles cannot simply be divided into growing and non-growing stages.
Treatment. Prior to any wine treatment, the specific nature of the reductive odor defect should be determined. Zoecklein et al (1999) and others recommend a simple aroma screen that can be used to differentiate hydrogen sulfide, mercaptans, and some oxidized VSC products.
Minimizing Reductive Odor Defect. The following considerations are important in minimizing reductive odor defect.
Adequate oxygen for yeasts. In addition to nitrogen, oxygen can be viewed as an important yeast nutrient (see “Factors, Considerations and Recommendations to Produce a Healthy Fermentation,” under “Factors Influencing Fermentation,” through On-line Publications at www.vtwines.info).
Juice turbidity. Turbidity of white juice should be adjusted to maintain the desirable aromatic finesse of the wine. Some winemakers measure the level of turbidity using a nephelometer. If the turbidity of the juice is too low (below 100 NTU), the concentration of N and other nutrients may be too low, increasing the likelihood of off-VSCs. If the turbidity of the juice is too high (greater than 300 NTU), there is a risk of the production of reductive odor defect from heavy sulfur-containing compounds, such as methionol, which is stable in wine and cannot be eliminated by aeration, racking, or copper.
Sulfur dioxide and oxygen. During fermentation, high levels of sulfur dioxide bind acetaldehyde, which is normally reduced to form ethanol. If not enough acetaldehyde is present, other juice components, such as sulfate, may be reduced instead, resulting in H2S formation. As such, the concentration of sulfur dioxide used pre-fermentation should be less than 8 g/hL.
Yeast lose their capacity to reduce sulfur, due to inactivation of the sulfite reductase enzyme (see above). It is only then that you would want to add sulfur dioxide. Adding sulfur dioxide prior to about 10 days after the completion of fermentation allows yeast to reduce the sulfite to sulfide, possibly increasing the hydrogen sulfide concentration.
Sulfur dioxide can be used to limit hydrogen sulfite in fermented wines. Sulfur dioxide can convert H2S to elemental sulfur as indicated in the reaction below. It should be noted that this reaction can go back the other way, if the wine is not carefully racked to remove the elemental sulfur (S).
SO2 + 2 H2S → 2 H2O + 3 S
Hydrogen sulfide can be removed by direct oxidation, according to the reaction below. While possible, this may have negative impacts on the wine quality via oxidative degradation.
2 H2S + O2 → 2 H2O + 2 S
One characteristic of sulfur odor defect is that these compounds are produced when the oxidation-reduction (redox) potential is low. One way to view oxidation–reduction is to substitute the term redox potential for the concentration of available oxygen. When that concentration is very low, there is a greater likelihood for the production of off sulfur-containing compounds. The presence of off-odor sulfur-containing compounds in a wine, and the corresponding smell, requires abnormally low oxygen concentrations (oxidation-reduction potential, values of –220 mV, compared to wine values of +220 to +450, when wine is exposed to air). This is the origin for the term reduced, which means a low redox value.
This is an important issue with regard to management. VSC problems are not as great in barreled wines, due to the higher oxygen (redox potential) which is maintained in barrels vs. tanks.
Frequent barrel stirring to put the lees in suspension, and limited oxidation across the staves, inhibits the formation of post-fermentation off-VSCs by increasing the oxidation-reduction values. This occurs at a more rapid rate in new wood, due to the greater oxygen dissolution and oxidizing effect of the new wood. During barrel aging, VSCs decrease progressively.
Lees management. It is clear that proper lees management is essential for minimizing the negative impact of VSCs. A relatively arbitrary but useful distinction can be made between heavy and light lees. Heavy lees are those that precipitate within 24 hours after the completion of yeast fermentation. Wines should be separated from heavy lees. Light lees, on the other hand, are those that precipitate later. These lees may be useful in improving a wine’s structural balance, texture, and antioxidant content, if properly managed.
If off-sulfur odors develop while on light lees, racking and aeration of the wine, with the temporary removal of the lees, has the advantage of providing some elimination of sulfur-containing compounds. Keeping the lees separate, with frequent stirring, and adding them back to the wine, has an added advantage. The lees can bind some VSCs, frequently eliminating the problem. Yeast walls can essentially act as fining agents, which can be an asset in management of sulfur compounds. The main proteins of the yeast cell wall have the ability to form disulfide bridges with some volatile sulfur compounds. If a newly-fermented wine has reductive odor defect, it is desirable to immediately separate it from the lees.
Copper Additions. Copper is permitted by the EEC at levels of 1 g/hL of copper sulfate, providing copper concentration is no more than 1 mg/L, and by the TTB to a level of 0.5 mg/L copper. H2S and mercaptans react with copper. Copper sulfide will not remove disulfides or heavy (high boiler) sulfur compounds.
Reaction of copper allows hydrogen sulfide to react with cupric sulfate to precipitate copper sulfide, according to the following:
H2S + CuSO4 → CuS + H2SO4
Oxidized volatile sulfur compounds, such as dimethyl disulfide, do not react directly with copper. In such cases, sulfur dioxide and ascorbic acid are used, according to the reaction below. The SO2 cleaves the disulfide, resulting in two mercaptans, which can then be bound with copper sulfide. The ascorbic acid acts as an antioxidant to keep the mercaptan from oxidizing. One of several problems is that this reaction is very slow at wine pHs.
Oxidation of methyl mercaptan to dimethyl disulfide, and reduction back to mercaptan, with the use of ascorbic acid:
2 CH3SH + ½ O2 → CH3SSCH3 + H2O
Methyl mercaptan Dimethyl disulfide
←
SO2/Ascorbic Acid
Adding copper sulfate during fermentation is a practice which some use to attempt to limit off-VSC impact on fermented wines. In such cases, the majority of the copper (about 60% or more) is bound to the yeast, and precipitates from solution. Copper addition, either during or post-fermentation, can have a large negative impact by lowering the varietal intensity of the aroma. Such additions lower varietal character notably in wines, such as Sauvignon blanc, where the varietal aromas are derived, in part, from sulfur-containing compounds.
Aeration. One of the potential problems of using aeration to lower the concentration of some low-boiling-point volatiles, like H2S and mercaptans, lies in the potential oxidation of mercaptans to disulfides (see above). To help avoid unwanted oxidation, especially in white wines, H2S may be blown off with inert gas, such as nitrogen. However, this may take a significant quantity of gas, and may result in aroma stripping.
It is essential that premium winemakers understand the nature of VSCs, and effective management procedures for dealing with reductive odor defect. Sulfur off-odors are dependent on the chemical nature of the particular compound, concentration, and wine. Problems can frequently be associated with certain vineyard blocks, low fermentable N, and the many factors that influence healthy yeast fermentations.
All wines should undergo an odor screen for reductive-odor defect before bottling (see Zoecklein et al., 1999).
The problem with off-VSCs is worldwide. Research needed in this area includes reviews of the qualitative and quantitative effects of vineyard management on N and sulfur-containing compounds. This includes sulfate uptake by vines, sulfur demand of vines, sulfur transport, and source-sink relationships, the influence of sulfur and nitrogen fertilization on grape and wine quality. The role of glutathione (a sulfur-containing polypeptide in the vines) needs further investigation (see Enology Notes #112).
![]()
Subscription to Enology Notes. All past Enology Notes newsjournals are posted on the Enology-Grape Chemistry Group's web site at: http://www.vtwines.info/.
To be added to (or removed from) the Enology Notes listserve send an email message to with the word "ADD" or "REMOVE" in the subject line.
Dr. Bruce
Zoecklein
Professor and Enology Specialist Head Enology-Grape Chemistry Group
Department of Food Science and Technology, Virginia Tech
Blacksburg VA 24061
Enology-Grape Chemistry Group Web address: http://www.vtwines.info/
Phone: (540) 231-5325
Fax: (540) 231-9293
Cell phone: 540-998-9025
Email:
